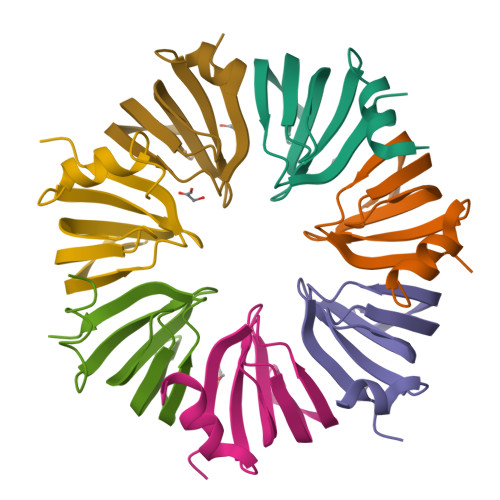
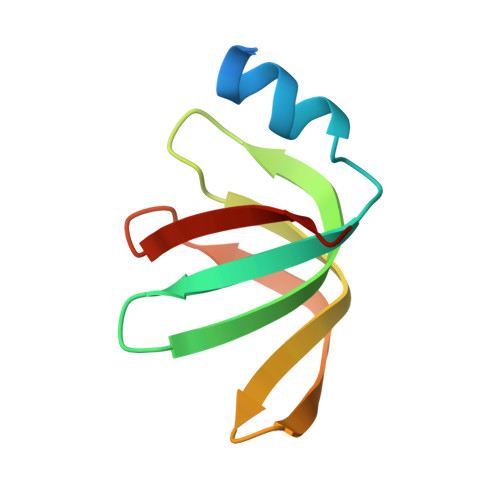
The crystal structure of a heptameric archaeal Sm protein: Implications for the eukaryotic snRNP core.
Mura, C., Cascio, D., Sawaya, M.R., Eisenberg, D.S.(2001) Proc Natl Acad Sci U S A 98: 5532-5537
- PubMed: 11331747
- DOI: https://doi.org/10.1073/pnas.091102298
- Primary Citation of Related Structures:
1I8F - PubMed Abstract:
Sm proteins form the core of small nuclear ribonucleoprotein particles (snRNPs), making them key components of several mRNA-processing assemblies, including the spliceosome. We report the 1.75-A crystal structure of SmAP, an Sm-like archaeal protein that forms a heptameric ring perforated by a cationic pore. In addition to providing direct evidence for such an assembly in eukaryotic snRNPs, this structure (i) shows that SmAP homodimers are structurally similar to human Sm heterodimers, (ii) supports a gene duplication model of Sm protein evolution, and (iii) offers a model of SmAP bound to single-stranded RNA (ssRNA) that explains Sm binding-site specificity. The pronounced electrostatic asymmetry of the SmAP surface imparts directionality to putative SmAP-RNA interactions.
Organizational Affiliation:
University of California at Los Angeles-Department of Energy Laboratory of Structural Biology and Molecular Medicine, 201 Boyer Hall/Molecular Biology Institute, Box 951570, Los Angeles, CA 90095-1570, USA.