Structural bases for sulfide recognition in Lucina pectinata hemoglobin I.
Rizzi, M., Wittenberg, J.B., Coda, A., Ascenzi, P., Bolognesi, M.(1996) J Mol Biol 258: 1-5
- PubMed: 8613980
- DOI: https://doi.org/10.1006/jmbi.1996.0228
- Primary Citation of Related Structures:
1MOH - PubMed Abstract:
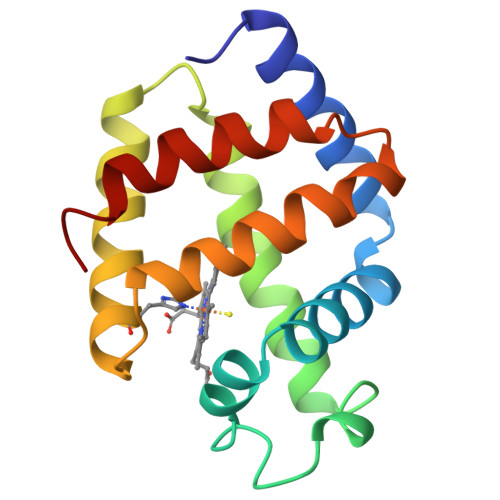
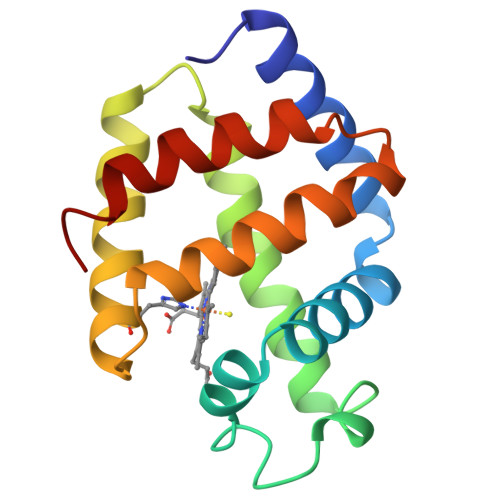
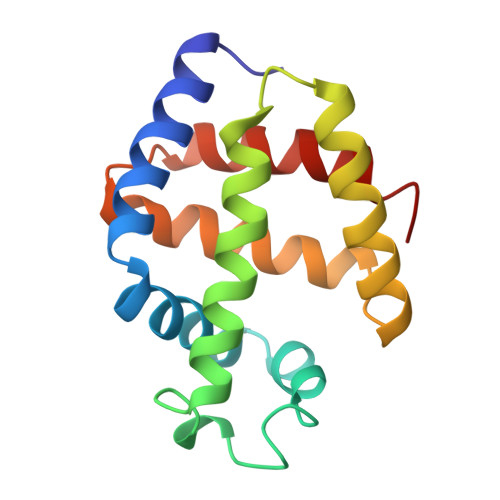
The X-ray crystal structure of the sulfide derivative of ferric Lucina pectinata hemoglobin component I (HbI) has been determined at 1.9 A resolution (R-factor 0.186). The heme pocket structural organization of HbI is in keeping with its ligand binding properties. The fast sulfide association rate constant can be related to the presence of Gln(64)E7, as the heme distal residue, together with the protein structural properties in the CD-E distal region. Moreover, the very high sulfide affinity for HbI is reflected by the exceptionally slow ligand dissociation rate. The stabilization of the heme-bound sulfide molecule is achieved through hydrogen bonding to Gln(64)E7, as well as by finely tuned aromatic-electrostatic interactions with the clustered residues Phe(29)B10, Phe(43)CD1 and Phe(68)E11. Such a peculiar arrangement of phenylalanyl residues at the distal ligand binding site has not been observed before in the globin family, and is unique to HbI, a protein functionally devoted to sulfide transport.
Organizational Affiliation:
Dipartimento di Genetica e Microbiologia, Università di Pavia, Italy.