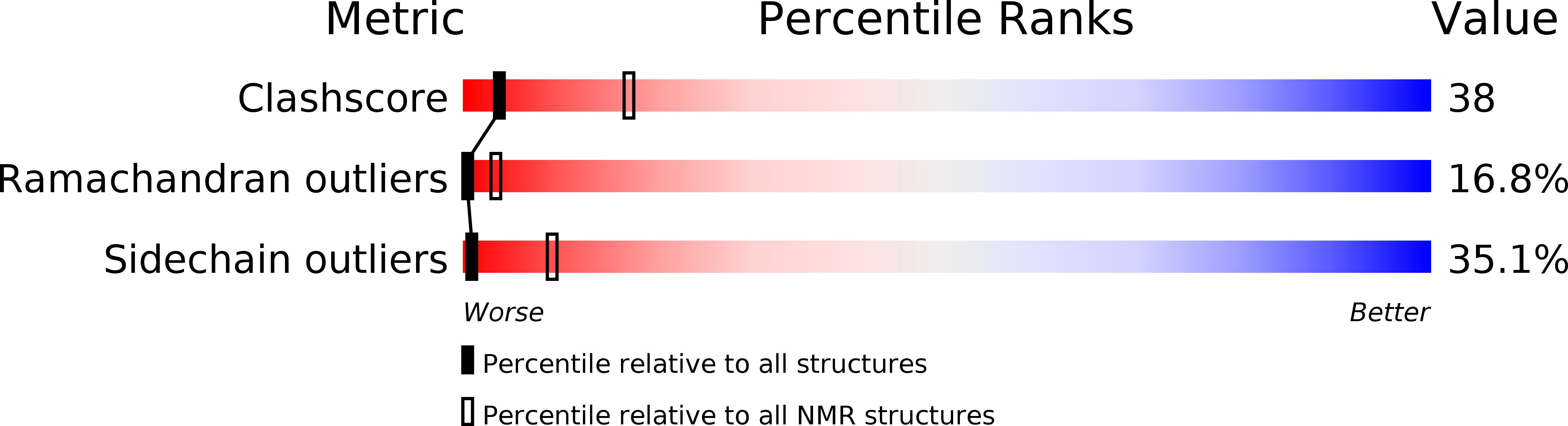The Structure of the Active Domain of the Herpes Simplex Virus Protein Icp47 in Water/Sodium Dodecyl Sulfate Solution Determined by Nuclear Magnetic Resonance Spectroscopy.
Pfaender, R., Neumann, L., Zweckstetter, M., Seger, C., Holak, T.A., Tampe, R.(1999) Biochemistry 38: 13692
- PubMed: 10521276
- DOI: https://doi.org/10.1021/bi9909647
- Primary Citation of Related Structures:
1QLO - PubMed Abstract:
ICP47 encoded by herpes simplex virus (HSV) is a key factor in the evasion of cellular immune response against HSV-infected cells. By specific inhibition of the transporter associated with antigen processing (TAP), ICP47 prevents peptide transport into the endoplasmic reticulum and subsequent loading of major histocompatibility complex (MHC) class I molecules. Amino acid residues 3-34 have been identified as the active domain. This domain appeared to be unstructured in aqueous solution, whereas after binding to membranes an alpha-helical conformation was observed. Here, we have analyzed the structure of ICP47(2-34) in a lipidlike environment by nuclear magnetic resonance (NMR) spectroscopy. In micellar solution of deuterated sodium dodecyl sulfate, the viral TAP inhibitor adopts an ordered structure. There are two helical regions extending from residues 4 to 15 and from residues 22 to 32. Arg-16 is found on the C-terminus of the first helix, and Gly-33 serves as a terminator of the second helix. A loop between residues 17 and 21 is also evident in the structure. The relative orientation of the helices toward each other, however, could not be determined due to the paucity of NOEs from residues 18-21.
Organizational Affiliation:
Max-Planck-Institut für Biochemie, Martinsried, Germany.