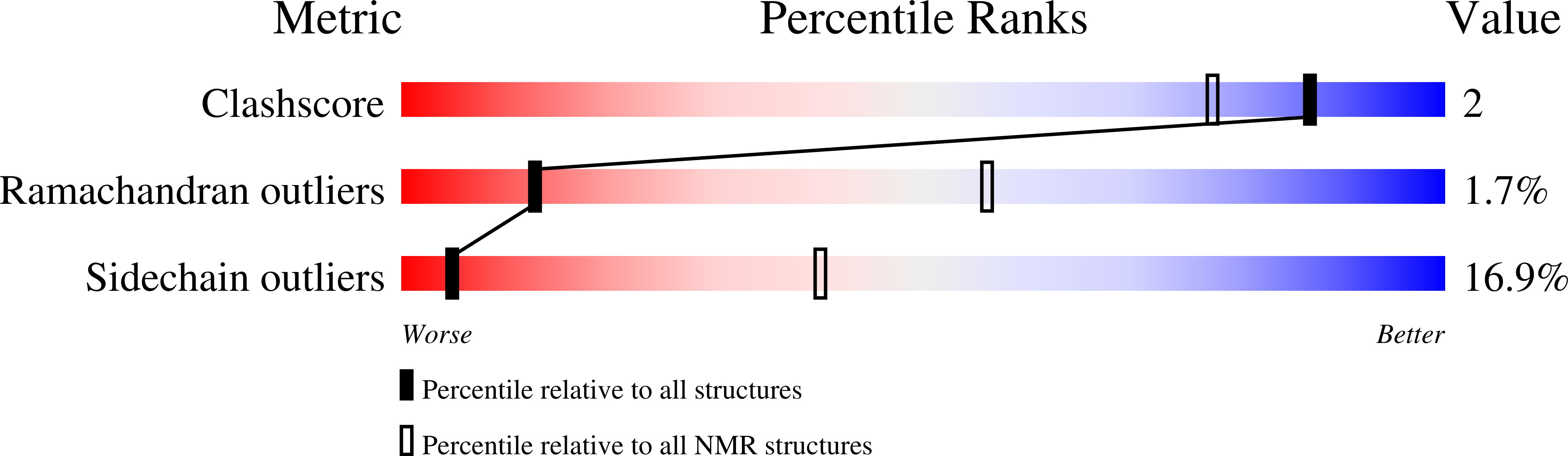Solution structures of the putative anti-sigma-factor antagonist TM1442 from Thermotoga maritima in the free and phosphorylated states.
Etezady-Esfarjani, T., Placzek, W.J., Herrmann, T., Wuthrich, K.(2006) Magn Reson Chem 44 Spec No: S61-S70
- PubMed: 16826544
- DOI: https://doi.org/10.1002/mrc.1831
- Primary Citation of Related Structures:
1SBO, 1T6R - PubMed Abstract:
The NMR structures of the unphosphorylated Thermotoga maritima protein TM1442 at pH 4.8 and of the phosphorylated TM1442 (TM1442-P) at pH 7.0 are presented, and a functional interaction of TM1442 with TM0733 is characterized. Although the NMR spectra of TM1442-P at pH 7.0 are of high quality, detailed NMR studies of unphosphorylated TM1442 could be performed only at slightly acidic pH values and high salt concentration. TM1442 is a putative anti-sigma-factor antagonist related to the sigmaF and sigmaB regulation systems in Bacillus subtilis, which is the component in this system that can be phosphorylated. The kinase TM0733, which shows sequence similarity to the GHKL ATPase/kinase superfamily, was identified as the possible anti-sigma-factor of TM1442 using a bioinformatics analysis. Phosphorylation of TM1442 by TM0733 was confirmed by NMR, mass spectroscopy and native gel electrophoresis, and Ser59 was identified as the phosphorylation site using site-directed mutational analysis. The solution structure of TM1442-P at pH 7.0 has the same global fold as free TM1442 at pH 4.8, with an alpha/beta topology consisting of a central four-stranded beta sheet and three alpha helices, but the regular secondary structure elements wrapping the hydrophobic core of the protein undergo subtle conformational changes upon phosphorylation.
Organizational Affiliation:
The Scripps Research Institute, Department of Molecular Biology and Joint Center for Structural Genomics, 10550 North Torrey Pines Road, La Jolla, CA 92037, USA. touraj@mol.bio.ethz.ch