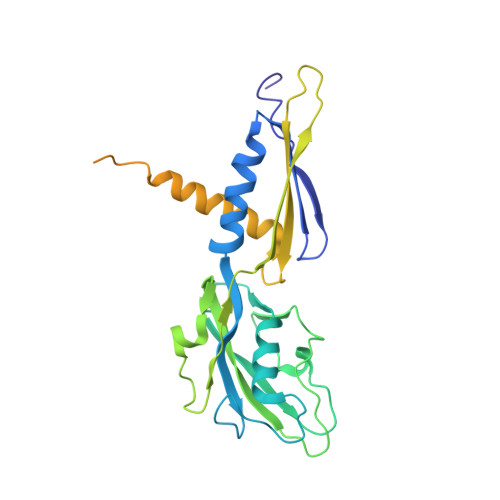
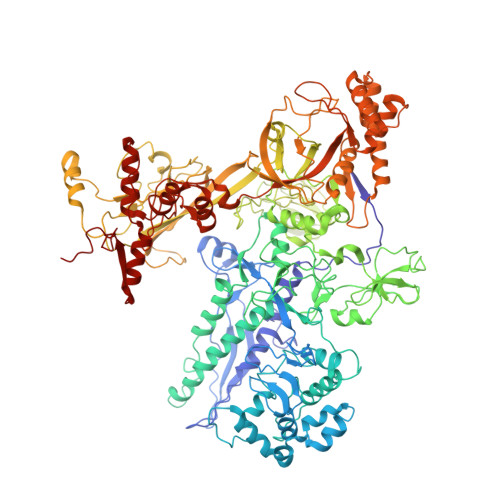
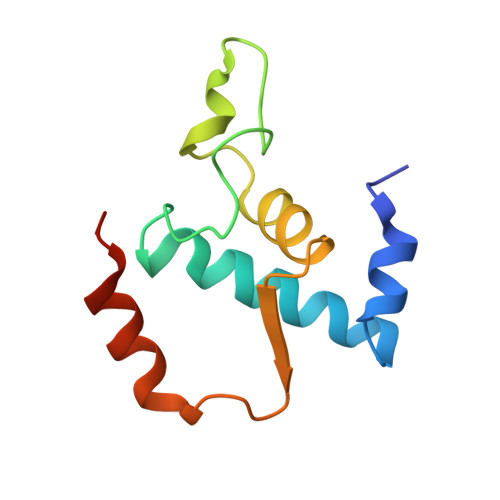
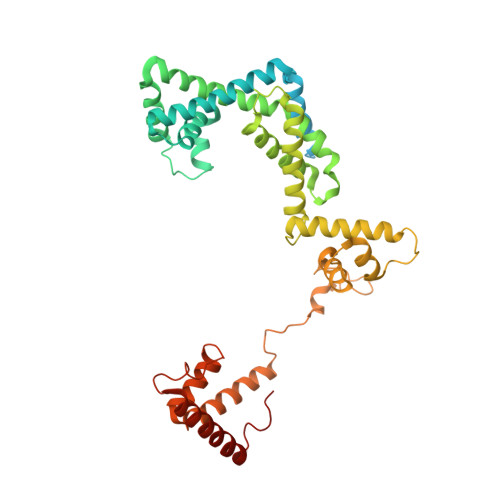
MG Query on MG Download Ideal Coordinates CCD File AA [auth A]
AA [auth A],
MAGNESIUM ION