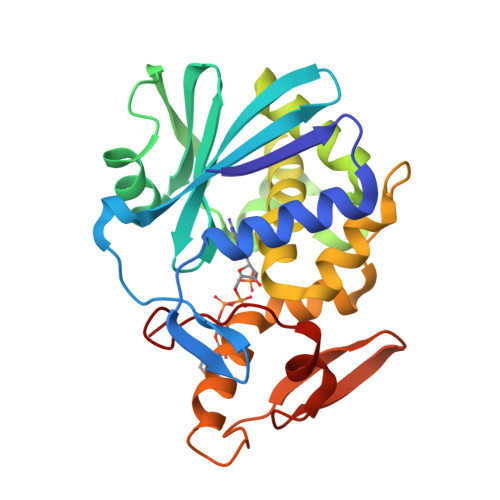
Crystal structure of trichosanthin-NADPH complex at 1.7 A resolution reveals active-site architecture.
Xiong, J.P., Xia, Z.X., Wang, Y.(1994) Nat Struct Biol 1: 695-700
- PubMed: 7634073
- DOI: https://doi.org/10.1038/nsb1094-695
- Primary Citation of Related Structures:
1TCS - PubMed Abstract:
We describe here the crystal structure of the trichosanthin-NADPH complex determined at a resolution of 1.7 A. The adenine base stacks between Tyr 70 and Tyr 111. Arg 163, Glu 160 and Tyr 70 form hydrogen bonds to N(3), O(3') and, through a water molecule, to N(9) of adenosine, respectively. This is the first high resolution structure of a complex between a ribosome-inactivating protein and a substrate analogue, in which the electron density of the N-glycosidic bond is well defined and the preassociated water, thought to be responsible for hydrolyzing the N-C bond, is also explicitly elucidated.
Organizational Affiliation:
Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences.