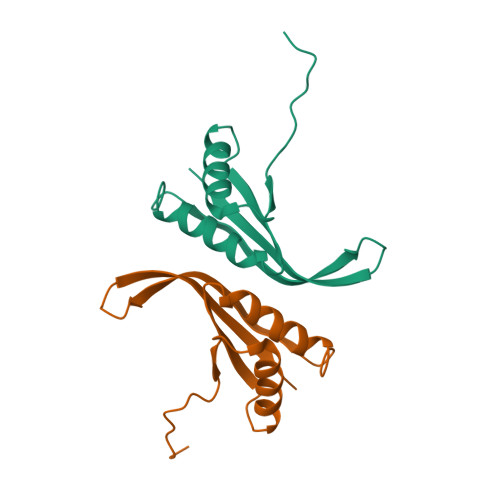
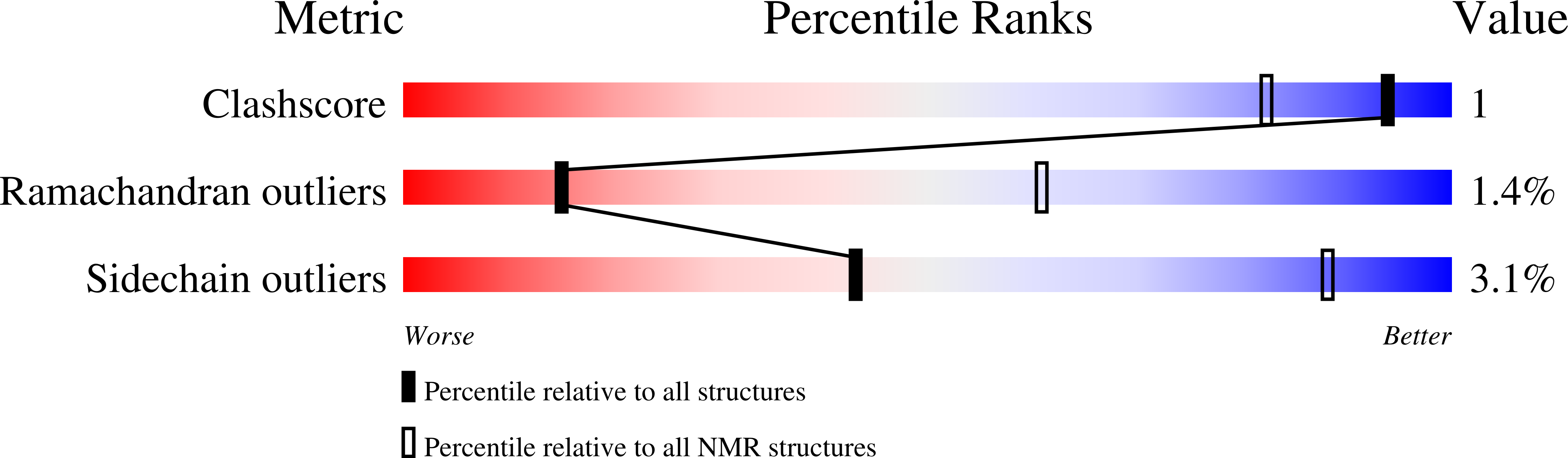A stabilizing alpha/beta-hydrophobic core greatly contributes to hyperthermostability of archaeal [P62A]Ssh10b.
Fang, X., Cui, Q., Tong, Y., Feng, Y., Shan, L., Huang, L., Wang, J.(2008) Biochemistry 47: 11212-11221
- PubMed: 18821773
- DOI: https://doi.org/10.1021/bi8007593
- Primary Citation of Related Structures:
1Y9X - PubMed Abstract:
The hyperthermophilic Ssh10b from Sulfolobus shibatae is a member of the Sac10b family, which has been postulated to play a role in chromosomal organization in Archaea. Ssh10b is capable of significantly constraining negative DNA supercoils at elevated temperatures. In this study, the solution structure of the dimeric P62A mutant Ssh10b ([P62A]Ssh10b) was determined by multidimensional NMR spectroscopy. The backbone 15N dynamics, H/D exchange with and without the denaturant GdmSCN, and chemical and thermal denaturation experiments were performed to investigate the molecular basis of high thermostability of [P62A]Ssh10b. Data analysis has revealed an alpha/beta-hydrophobic core consisting of two alpha-helices and one beta-sheet which are stabilized by cooperative hydrophobic and hydrogen-bonding interactions. This stabilizing alpha/beta-hydrophobic core of [P62A]Ssh10b exhibiting highly restricted internal motions is composed of residues having highly protected amide protons which exchange with solvent mostly by means of a global unfolding process. The K40N mutation greatly destabilizes the mutant [P62A]Ssh10b because this mutation disturbs the packing of alpha-helix against the beta-sheet reducing the stability of the alpha/beta-hydrophobic core in the mutant protein. In comparison with homologous mesophilic and thermophilic proteins, it can be presumed that the stabilizing alpha/beta-hydrophobic core in the [P62A]Ssh10b structure greatly contributes to the high thermostability of the protein.
Organizational Affiliation:
National Laboratory of Biomacromolecules, Institute of Biophysics, Chinese Academy of Sciences, 15 Datun Road, Beijing 100101, China.