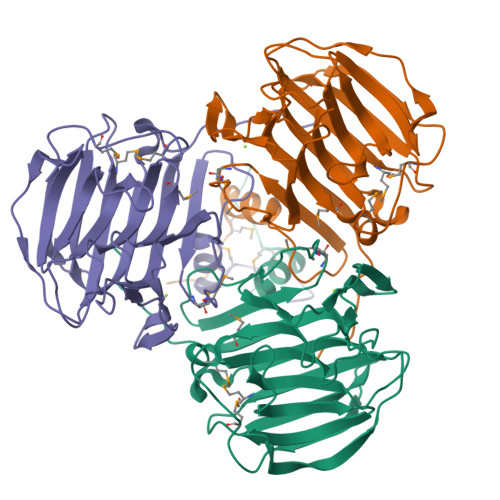
Structure of SO2946 orphan from Shewanella oneidensis shows "jelly-roll" fold with carbohydrate-binding module.
Nocek, B., Bigelow, L., Abdullah, J., Joachimiak, A.(2008) J Struct Funct Genomics 9: 1-6
- PubMed: 18566914
- DOI: https://doi.org/10.1007/s10969-008-9040-0
- Primary Citation of Related Structures:
2A5Z - PubMed Abstract:
The crystal structure of the uncharacterized protein SO2946 from Shewanella oneidensis MR-1 was determined with single-wavelength anomalous diffraction (SAD) and refined to 2.0 A resolution. The SO2946 protein consists of a short helical N-terminal domain and a large C-terminal domain with the "jelly-roll" topology. The protein assembles into a propeller consisting of three C-terminal blades arranged around a central core formed by the N-terminal domains. The function of SO2946 could not be inferred from the sequence since the protein represents an orphan with no sequence homologs, but the protein's structure bears a fold similar to that of proteins containing carbohydrate-binding modules. Features such as fold conservation, the presence of a conserved groove and a metal binding region are indicative that SO2946 may be an enzyme and could be involved in binding carbohydrate molecules.
Organizational Affiliation:
Midwest Center for Structural Genomics, Biosciences Division, Argonne National Laboratory, 9700 South Cass Avenue, Building 202, Argonne, IL 60439, USA.