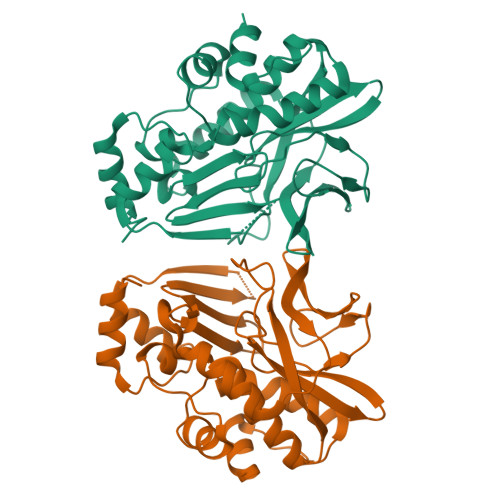
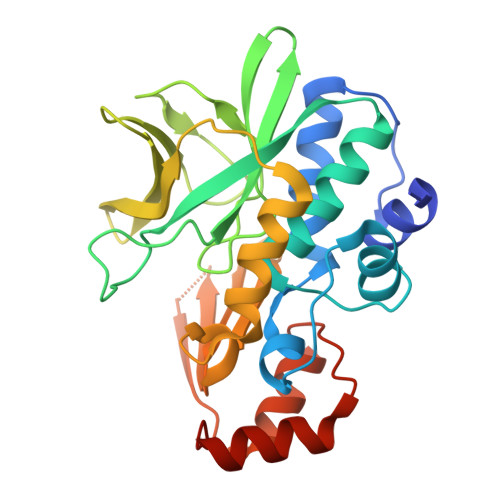
Structure of Mesorhizobium Loti Arylamine N-Acetyltransferase 1.
Holton, S.J., Dairou, J., Sandy, J., Rodrigues-Lima, F., Dupret, J.M., Noble, M.E.M., Sim, E.(2005) Acta Crystallogr Sect F Struct Biol Cryst Commun 61: 14
- PubMed: 16508079
- DOI: https://doi.org/10.1107/S1744309104030659
- Primary Citation of Related Structures:
2BSZ - PubMed Abstract:
The arylamine N-acetyltransferase (NAT) enzymes have been found in a broad range of both eukaryotic and prokaryotic organisms. The NAT enzymes catalyse the transfer of an acetyl group from acetyl Co-enzyme A onto the terminal nitrogen of a range of arylamine, hydrazine and arylhydrazine compounds. Recently, several NAT structures have been reported from different prokaryotic sources including Salmonella typhimurium, Mycobacterium smegmatis and Pseudomonas aeruginosa. Bioinformatics analysis of the Mesorhizobium loti genome revealed two NAT paralogues, the first example of multiple NAT isoenzymes in a eubacterial organism. The M. loti NAT 1 enzyme was recombinantly expressed and purified for X-ray crystallographic studies. The purified enzyme was crystallized in 0.5 M Ca(OAc)2, 16% PEG 3350, 0.1 M Tris-HCl pH 8.5 using the sitting-drop vapour-diffusion method. A data set diffracting to 2.0 A was collected from a single crystal at 100 K. The crystal belongs to the orthorhombic spacegroup P2(1)2(1)2(1), with unit-cell parameters a = 53.2, b = 97.3, c = 114.3 A. The structure was refined to a final free-R factor of 24.8%. The structure reveals that despite low sequence homology, M. loti NAT1 shares the common fold as reported in previous NAT structures and exhibits the same catalytic triad of residues (Cys-His-Asp) in the active site.
Organizational Affiliation:
Department of Biochemistry, Oxford University, Oxford OX1 3QU, England.