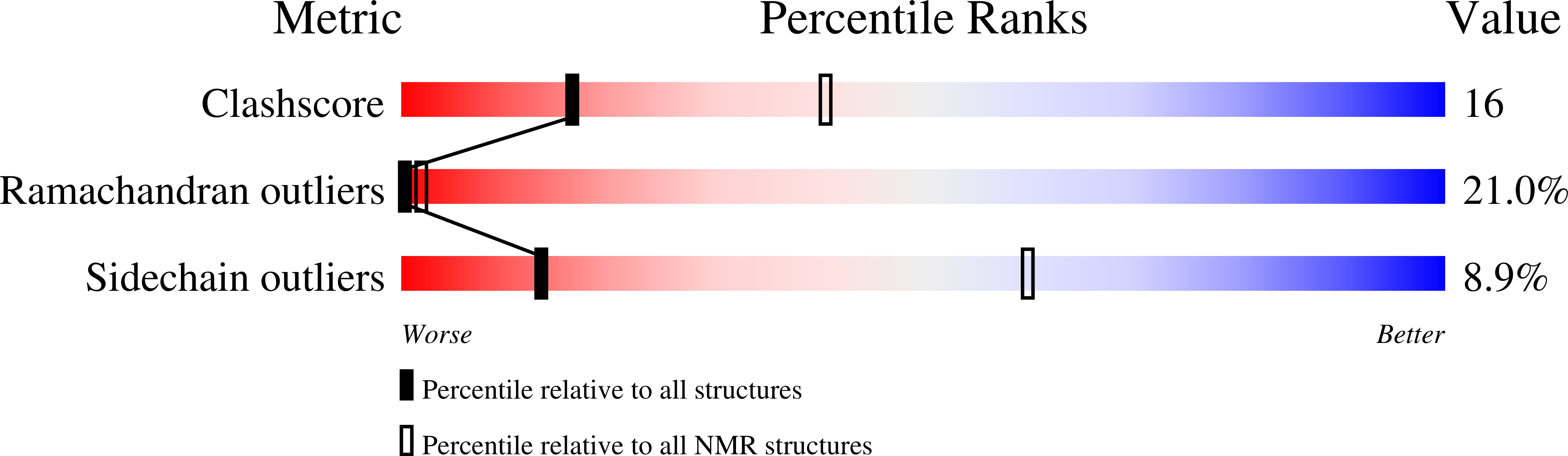Identification and structural determination of the M3 muscarinic acetylcholine receptor basolateral sorting signal.
Iverson, H.A., Fox, D., Nadler, L.S., Klevit, R.E., Nathanson, N.M.(2005) J Biol Chem 280: 24568-24575
- PubMed: 15870063
- DOI: https://doi.org/10.1074/jbc.M501264200
- Primary Citation of Related Structures:
2CSA - PubMed Abstract:
Muscarinic acetylcholine receptors comprise a family of G-protein-coupled receptors that display differential localization in polarized epithelial cells. We identify a seven-residue sequence, Ala(275)-Val(281), in the third intracellular loop of the M(3) muscarinic receptor that mediates dominant, position-independent basolateral targeting in Madin-Darby canine kidney cells. Mutational analyses identify Glu(276), Phe(280), and Val(281) as critical residues within this sorting motif. Phe(280) and Val(281) comprise a novel dihydrophobic sorting signal as mutations of either residue singly or together with leucine do not disrupt basolateral targeting. Conversely, Glu(276) is required and cannot be substituted with alanine or aspartic acid. A 19-amino acid peptide representing the M(3) sorting signal and surrounding sequence was analyzed via two-dimensional nuclear magnetic resonance spectroscopy. Solution structures show that Glu(276) resides in a type IV beta-turn and the dihydrophobic sequence Phe(280)Val(281) adopts either a type I or IV beta-turn.
Organizational Affiliation:
Department of Pharmacology, University of Washington, Seattle, Washington 98195, USA.