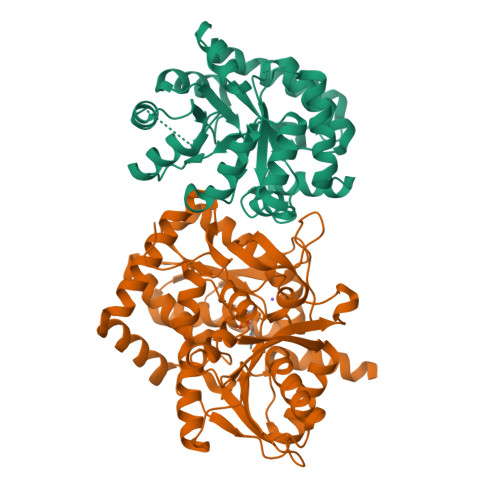
Betaq114N and Betat110V Mutations Reveal a Critically Important Role of the Substrate Alpha-Carboxylate Site in the Reaction Specificity of Tryptophan Synthase.
Blumenstein, L., Domratcheva, T., Niks, D., Ngo, H., Seidel, R., Dunn, M.F., Schlichting, I.(2007) Biochemistry 46: 14100
- PubMed: 18004874
- DOI: https://doi.org/10.1021/bi7008568
- Primary Citation of Related Structures:
2J9Y, 2J9Z - PubMed Abstract:
In the PLP-requiring alpha2beta2 tryptophan synthase complex, recognition of the substrate l-Ser at the beta-site includes a loop structure (residues beta110-115) extensively H-bonded to the substrate alpha-carboxylate. To investigate the relationship of this subsite to catalytic function and to the regulation of substrate channeling, two loop mutants were constructed: betaThr110 --> Val, and betaGln114 --> Asn. The betaT110V mutation greatly impairs both catalytic activity in the beta-reaction, and allosteric communication between the alpha- and beta-sites. The crystal structure of the betaT110V mutant shows that the modified l-Ser carboxylate subsite has altered protein interactions that impair beta-site catalysis and the communication of allosteric signals between the alpha- and beta-sites. Purified betaQ114N consists of two species of mutant protein, one with a reddish color (lambdamax = 506 nm). The reddish species is unable to react with l-Ser. The second betaQ114N species displays significant catalytic activities; however, intermediates obtained on reaction with substrate l-Ser and substrate analogues exhibit perturbed UV/vis absorption spectra. Incubation with l-Ser results in the formation of an inactive species during the first 15 min with lambdamax approximately 320 nm, followed by a slower conversion over 24 h to the species with lambdamax = 506 nm. The 320 and 506 nm species originate from conversion of the alpha-aminoacrylate external aldimine to the internal aldimine and alpha-aminoacrylate, followed by the nucleophilic attack of alpha-aminoacrylate on C-4' of the internal aldimine to give a covalent adduct with PLP. Subsequent treatment with sodium hydroxide releases a modified coenzyme consisting of a vinylglyoxylic acid moiety linked through C-4' to the 4-position of the pyridine ring. We conclude that the shortening of the side chain accompanying the replacement of beta114-Gln by Asn relaxes the steric constraints that prevent this reaction in the wild-type enzyme. This study reveals a new layer of structure-function interactions essential for reaction specificity in tryptophan synthase.
Organizational Affiliation:
Max Planck Institute for Medical Research, Department of Biomolecular Mechanisms, Heidelberg, Germany.