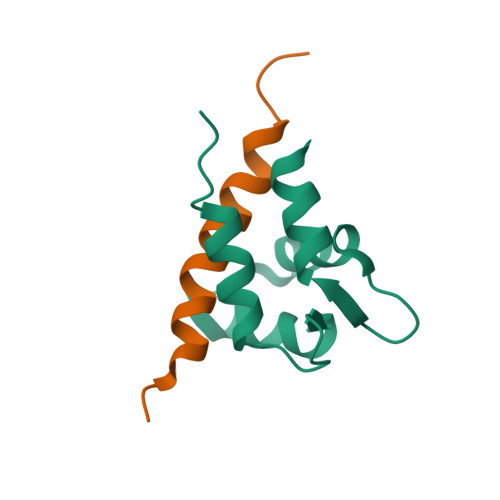
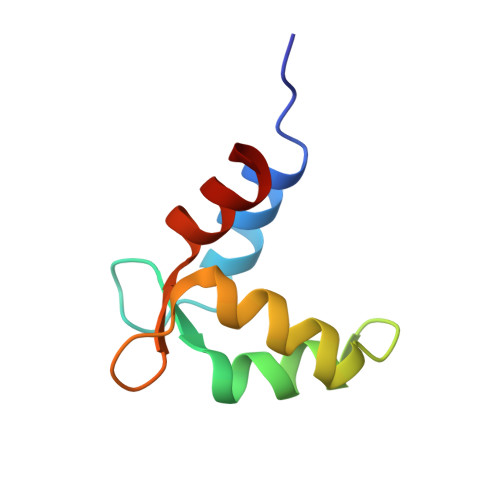
Structural and Energetic Determinants of Apo Calmodulin Binding to the IQ Motif of the Na(V)1.2 Voltage-Dependent Sodium Channel.
Feldkamp, M.D., Yu, L., Shea, M.A.(2011) Structure 19: 733-747
- PubMed: 21439835
- DOI: https://doi.org/10.1016/j.str.2011.02.009
- Primary Citation of Related Structures:
2KXW - PubMed Abstract:
The neuronal voltage-dependent sodium channel (Na(v)1.2), essential for generation and propagation of action potentials, is regulated by calmodulin (CaM) binding to the IQ motif in its α subunit. A peptide (Na(v)1.2(IQp), KRKQEEVSAIVIQRAYRRYLLKQKVKK) representing the IQ motif had higher affinity for apo CaM than (Ca(2+))(4)-CaM. Association was mediated solely by the C-domain of CaM. A solution structure (2KXW.pdb) of apo (13)C,(15)N-CaM C-domain bound to Na(v)1.2(IQp) was determined with NMR. The region of Na(v)1.2(IQp) bound to CaM was helical; R1902, an Na(v)1.2 residue implicated in familial autism, did not contact CaM. The apo C-domain of CaM in this complex shares features of the same domain bound to myosin V IQ motifs (2IX7) and bound to an SK channel peptide (1G4Y) that does not contain an IQ motif. Thermodynamic and structural studies of CaM-Na(v)1.2(IQp) interactions show that apo and (Ca(2+))(4)-CaM adopt distinct conformations that both permit tight association with Na(v)1.2(IQp) during gating.
Organizational Affiliation:
Department of Biochemistry, Roy J. and Lucille A. Carver College of Medicine, University of Iowa, Iowa City, IA 52242-1109, USA.