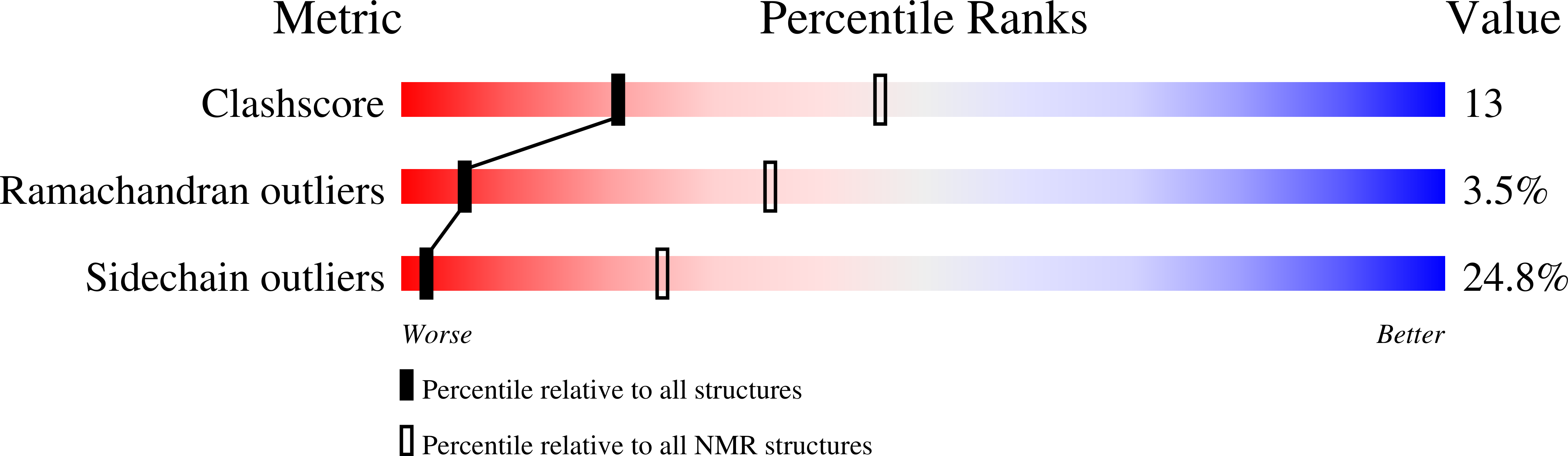The Receptor for Advanced Glycation End Products (RAGE) Specifically Recognizes Methylglyoxal-Derived AGEs.
Xue, J., Ray, R., Singer, D., Bohme, D., Burz, D.S., Rai, V., Hoffmann, R., Shekhtman, A.(2014) Biochemistry 53: 3327-3335
- PubMed: 24824951
- DOI: https://doi.org/10.1021/bi500046t
- Primary Citation of Related Structures:
2MOV - PubMed Abstract:
Diabetes-induced hyperglycemia increases the extracellular concentration of methylglyoxal. Methylglyoxal-derived hydroimidazolones (MG-H) form advanced glycation end products (AGEs) that accumulate in the serum of diabetic patients. The binding of hydroimidozolones to the receptor for AGEs (RAGE) results in long-term complications of diabetes typified by vascular and neuronal injury. Here we show that binding of methylglyoxal-modified albumin to RAGE results in signal transduction. Chemically synthesized peptides containing hydroimidozolones bind specifically to the V domain of RAGE with nanomolar affinity. The solution structure of an MG-H1-V domain complex revealed that the hydroimidazolone moiety forms multiple contacts with a positively charged surface on the V domain. The high affinity and specificity of hydroimidozolones binding to the V domain of RAGE suggest that they are the primary AGE structures that give rise to AGEs-RAGE pathologies.
Organizational Affiliation:
Department of Chemistry, State University of New York at Albany , Albany, New York 12222, United States.