Structure-based drug design of pyrrolidine-1, 2-dicarboxamides as a novel series of orally bioavailable factor Xa inhibitors
Van Huis, C.A., Bigge, C.F., Casimiro-Garcia, A., Cody, W.L., Dudley, D.A., Filipski, K.J., Heemstra, R.J., Kohrt, J.T., Narasimhan, L.S., Schaum, R.P., Zhang, E., Bryant, J.W., Haarer, S., Janiczek, N., Leadley, R.J., McClanahan, T., Thomas Peterson, J., Welch, K.M., Edmunds, J.J.(2007) Chem Biol Drug Des 69: 444-450
- PubMed: 17581239
- DOI: https://doi.org/10.1111/j.1747-0285.2007.00520.x
- Primary Citation of Related Structures:
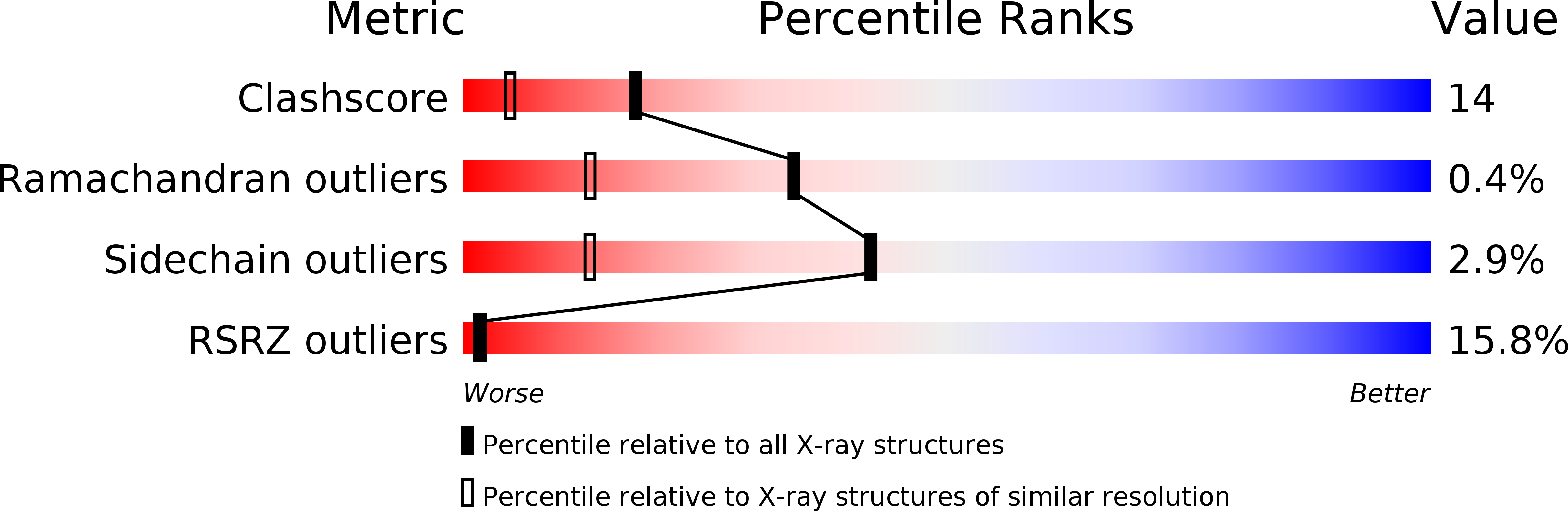
2PR3 - PubMed Abstract:
A novel series of pyrrolidine-1,2-dicarboxamides was discovered as factor Xa inhibitors using structure-based drug design. This series consisted of a neutral 4-chlorophenylurea P1, a biphenylsulfonamide P4 and a D-proline scaffold (1, IC(50) = 18 nM). Optimization of the initial hit resulted in an orally bioavailable, subnanomolar inhibitor of factor Xa (13, IC(50) = 0.38 nM), which was shown to be efficacious in a canine electrolytic model of thrombosis with minimal bleeding.