Structure-function relationships in a bacterial DING protein.
Ahn, S., Moniot, S., Elias, M., Chabriere, E., Kim, D., Scott, K.(2007) FEBS Lett 581: 3455-3460
- PubMed: 17612529
- DOI: https://doi.org/10.1016/j.febslet.2007.06.050
- Primary Citation of Related Structures:
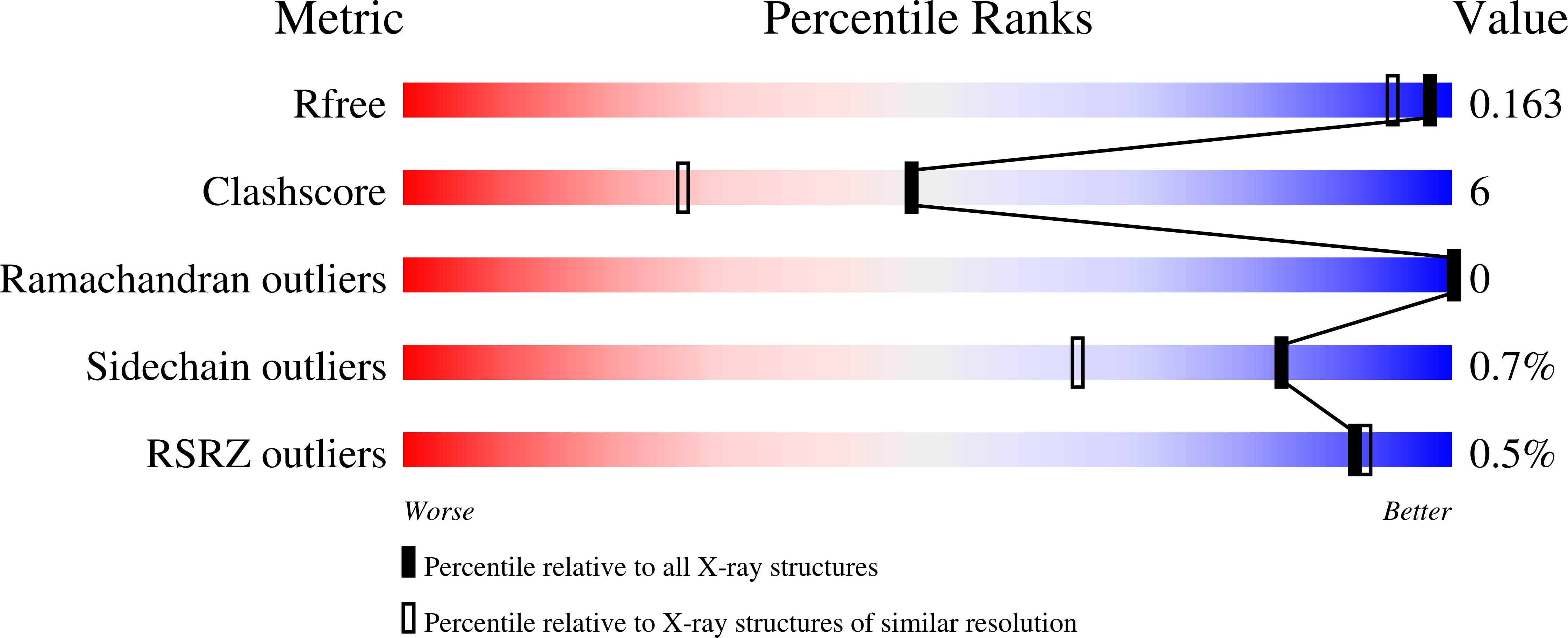
2Q9T - PubMed Abstract:
A recombinant DING protein from Pseudomonas fluorescens has been previously shown to have a phosphate-binding site, and to be mitogenic for human cells. Here we report the three-dimensional structure of the protein, confirming a close similarity to the "Venus flytrap" structure seen in other human and bacterial phosphate-binding proteins. Site-directed mutagenesis confirms the role of a key residue involved in phosphate binding, and that the mitogenic activity is not dependent on this property. Deletion of one of the two hinged domains that constitute the Venus flytrap also eliminates phosphate binding whilst enhancing mitogenic activity.
Organizational Affiliation:
School of Biological Sciences, The University of Auckland, Auckland, New Zealand.