Potent D-peptide inhibitors of HIV-1 entry
Welch, B.D., Vandemark, A.P., Heroux, A., Hill, C.P., Kay, M.S.(2007) Proc Natl Acad Sci U S A 104: 16828-16833
- PubMed: 17942675
- DOI: https://doi.org/10.1073/pnas.0708109104
- Primary Citation of Related Structures:
2R3C, 2R5B, 2R5D - PubMed Abstract:
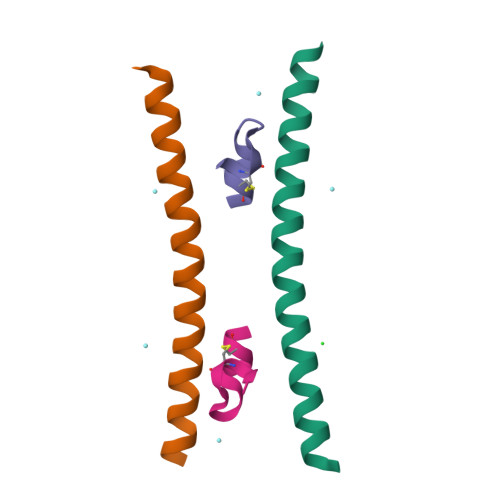
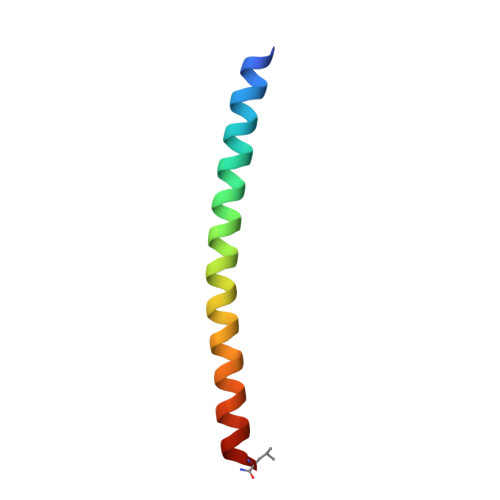
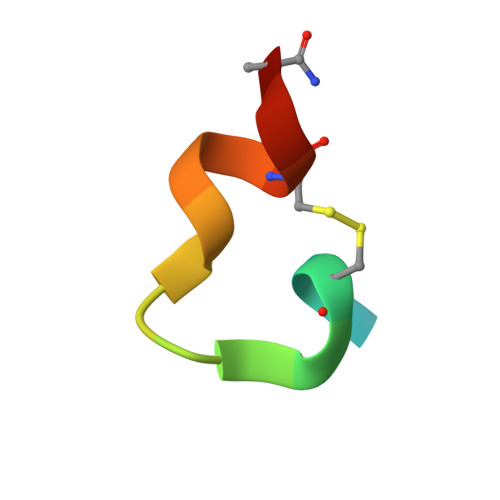
During HIV-1 entry, the highly conserved gp41 N-trimer pocket region becomes transiently exposed and vulnerable to inhibition. Using mirror-image phage display and structure-assisted design, we have discovered protease-resistant D-amino acid peptides (D-peptides) that bind the N-trimer pocket with high affinity and potently inhibit viral entry. We also report high-resolution crystal structures of two of these D-peptides in complex with a pocket mimic that suggest sources of their high potency. A trimeric version of one of these peptides is the most potent pocket-specific entry inhibitor yet reported by three orders of magnitude (IC(50) = 250 pM). These results are the first demonstration that D-peptides can form specific and high-affinity interactions with natural protein targets and strengthen their promise as therapeutic agents. The D-peptides described here address limitations associated with current L-peptide entry inhibitors and are promising leads for the prevention and treatment of HIV/AIDS.
Organizational Affiliation:
Department of Biochemistry, University of Utah, Emma Eccles Jones Medical Research Building, 15 North Medical Drive East, Salt Lake City, UT 84112-5650, USA.