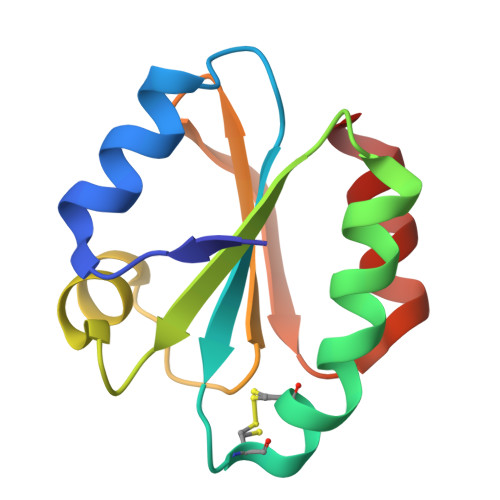
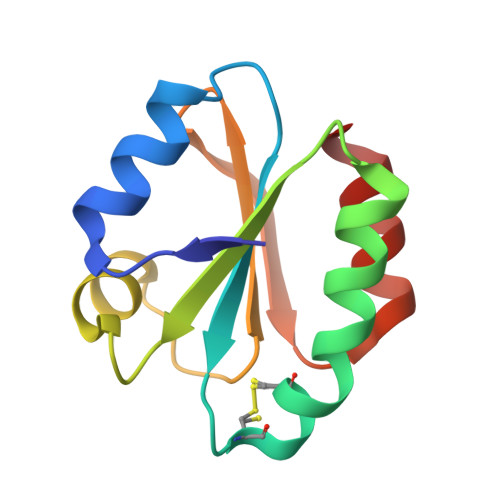
The Fasciola Hepatica Thioredoxin: High Resolution Structure Reveals Two Oxidation States.
Line, K., Isupov, M.N., Garcia-Rodriguez, E., Maggioli, G., Parra, F., Littlechild, J.A.(2008) Mol Biochem Parasitol 161: 44
- PubMed: 18620002
- DOI: https://doi.org/10.1016/j.molbiopara.2008.06.009
- Primary Citation of Related Structures:
2VIM - PubMed Abstract:
The Fasciola hepatica thioredoxin protein structure has been determined to 1.45A resolution. This is the first example of a single crystal structure to show the active site cysteine residues in both the reduced and disulfide oxidised form. Consistent with this observation the process of oxidation appears to require very little rearrangement of the surrounding protein structure. The F. hepatica thioredoxin structure has been compared to other thioredoxin protein structures already known and is found to be highly conserved. The F. hepatica protein is most similar to that of the thioredoxin from its human and animal hosts but it resembles other parasitic thioredoxins with regard to having no additional cysteine residues and is therefore not regulated by transient disulfide bond formation as proposed for thioredoxins from higher eukaryotic species.
Organizational Affiliation:
Henry Wellcome Building for Biocatalysis, School of Biosciences, University of Exeter, Stocker Road, Exeter EX4 4QD, UK.