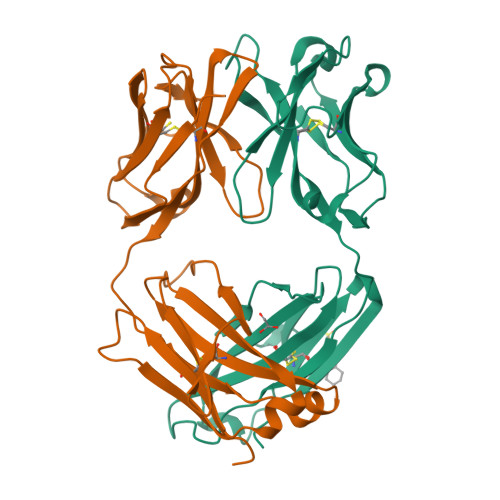
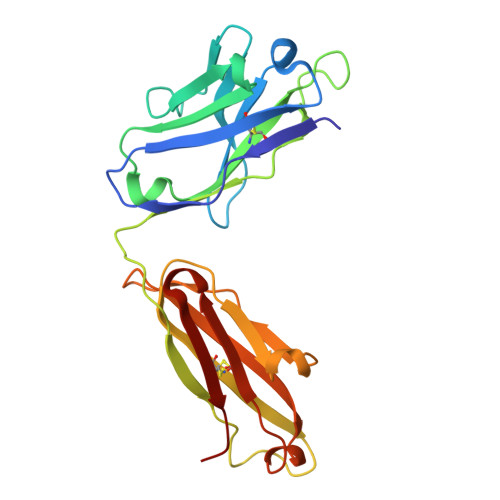
Unusual Water-Mediated Antigenic Recognition of the Proinflammatory Cytokine Interleukin-18.
Argiriadi, M.A., Xiang, T., Wu, C., Ghayur, T., Borhani, D.W.(2009) J Biol Chem 284: 24478
- PubMed: 19553661
- DOI: https://doi.org/10.1074/jbc.M109.023887
- Primary Citation of Related Structures:
2VXT, 2VXU, 2VXV - PubMed Abstract:
The unique cytokine interleukin-18 (IL-18) acts synergistically with IL-12 to regulate T-helper 1 and 2 lymphocytes and, as such, seems to underlie the pathogenesis of various autoimmune and allergic diseases. Several anti-IL-18 agents are in clinical development, including the recombinant human antibody ABT-325, which is entering trials for autoimmune diseases. Given competing cytokine/receptor and cytokine/receptor decoy interactions, understanding the structural basis for recognition is critical for effective development of anti-cytokine therapies. Here we report three crystal structures: the murine antibody 125-2H Fab fragment bound to human IL-18, at 1.5 A resolution; the 125-2H Fab (2.3 A); and the ABT-325 Fab (1.5 A). These structures, along with human/mouse IL-18 chimera binding data, allow us to make three key observations relevant to the biology and antigenic recognition of IL-18 and related cytokines. First, several IL-18 residues shift dramatically (> 10 A) upon binding 125-2H, compared with unbound IL-18 (Kato, Z., Jee, J., Shikano, H., Mishima, M., Ohki, I., Ohnishi, H., Li, A., Hashimoto, K., Matsukuma, E., Omoya, K., Yamamoto, Y., Yoneda, T., Hara, T., Kondo, N., and Shirakawa, M. (2003) Nat. Struct. Biol. 10, 966-971). IL-18 thus exhibits plasticity that may be common to its interactions with other receptors. Related cytokines may exhibit similar plasticity. Second, ABT-325 and 125-2H differ significantly in combining site character and architecture, thus explaining their ability to bind IL-18 simultaneously at distinct epitopes. These data allow us to define the likely ABT-325 epitope and thereby explain the distinct neutralizing mechanisms of both antibodies. Third, given the high 125-2H potency, 10 well ordered water molecules are trapped upon complex formation in a cavity between two IL-18 loops and all six 125-2H complementarity-determining regions. Thus, counterintuitively, tight and specific antibody binding may in some cases be water-mediated.
Organizational Affiliation:
Department of Biochemistry, Abbott Laboratories, Worcester, Massachusetts 01605, USA. maria.argiriadi@abbott.com