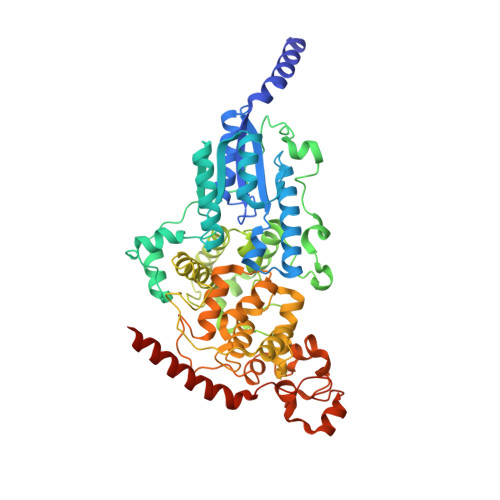
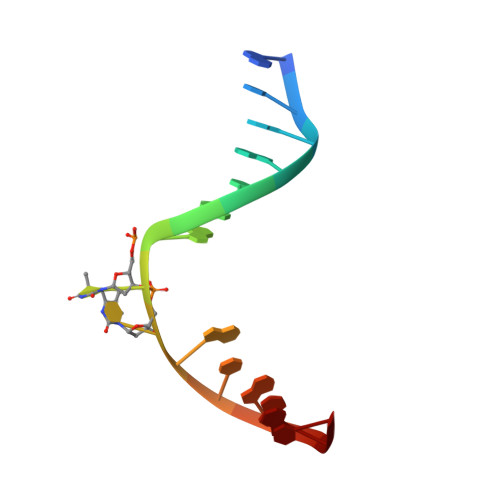
The archaeal cofactor F0 is a light-harvesting antenna chromophore in eukaryotes.
Glas, A.F., Maul, M.J., Cryle, M., Barends, T.R., Schneider, S., Kaya, E., Schlichting, I., Carell, T.(2009) Proc Natl Acad Sci U S A 106: 11540-11545
- PubMed: 19570997
- DOI: https://doi.org/10.1073/pnas.0812665106
- Primary Citation of Related Structures:
3CVV - PubMed Abstract:
Archae possess unique biochemical systems quite distinct from the pathways present in eukaryotes and eubacteria. 7,8-Dimethyl-8-hydroxy-5deazaflavin (F(0)) and F(420) are unique deazaflavin-containing coenzyme and methanogenic signature molecules, essential for a variety of biochemical transformations associated with methane biosynthesis and light-dependent DNA repair. The deazaflavin cofactor system functions during methane biosynthesis as a low-potential hydrid shuttle F(420)/F(420)H(2). In DNA photolyase repair proteins, the deazaflavin cofactor is in the deprotonated state active as a light-collecting energy transfer pigment. As such, it converts blue sunlight into energy used by the proteins to drive an essential repair process. Analysis of a eukaryotic (6-4) DNA photolyase from Drosophila melanogaster revealed a binding pocket, which tightly binds F(0). Residues in the pocket activate the cofactor by deprotonation so that light absorption and energy transfer are switched on. The crystal structure of F(0) in complex with the D. melanogaster protein shows the atomic details of F(0) binding and activation, allowing characterization of the residues involved in F(0) activation. The results show that the F(0)/F(420) coenzyme system, so far believed to be strictly limited to the archael kingdom of life, is far more widespread than anticipated. Analysis of a D. melanogaster extract and of a DNA photolyase from the primitive eukaryote Ostreococcus tauri provided direct proof for the presence of the F(0) cofactor also in higher eukaryotes.
- Department of Chemistry and Biochemistry, Ludwig-Maximilians University Munich, Centre for Integrative Protein Science, 81377 Munich, Germany.
Organizational Affiliation: