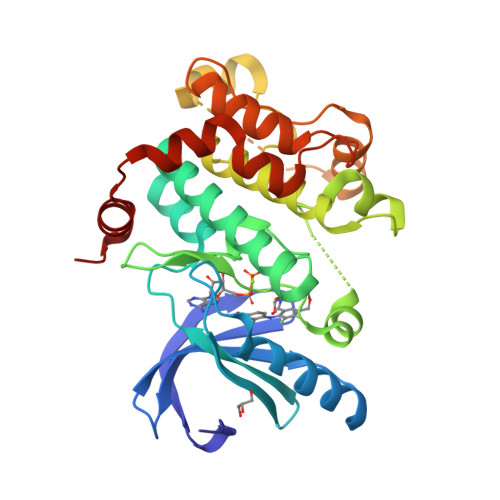
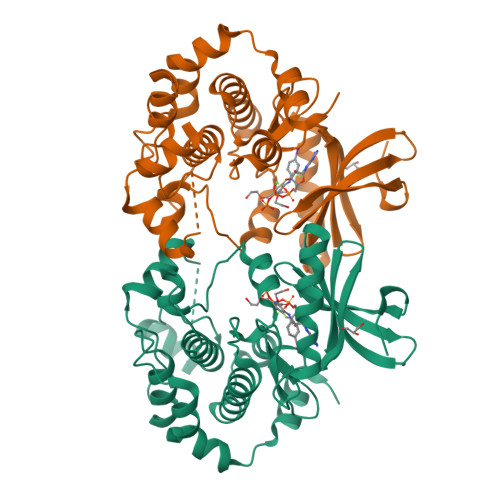
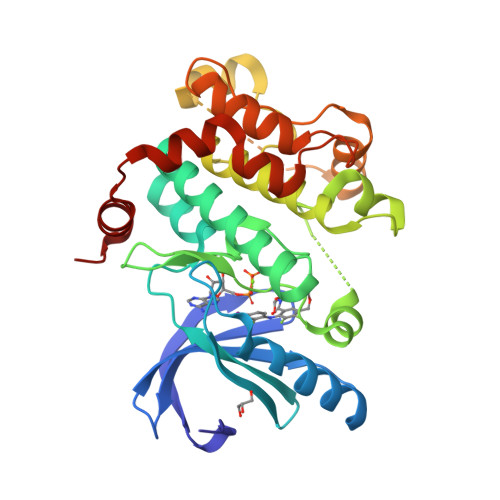
Beyond the MEK-pocket: can current MEK kinase inhibitors be utilized to synthesize novel type III NCKIs? Does the MEK-pocket exist in kinases other than MEK?
Tecle, H., Shao, J., Li, Y., Kothe, M., Kazmirski, S., Penzotti, J., Ding, Y.H., Ohren, J., Moshinsky, D., Coli, R., Jhawar, N., Bora, E., Jacques-O'Hagan, S., Wu, J.(2009) Bioorg Med Chem Lett 19: 226-229
- PubMed: 19019675
- DOI: https://doi.org/10.1016/j.bmcl.2008.10.108
- Primary Citation of Related Structures:
3DV3, 3DY7 - PubMed Abstract:
An approach and preliminary results for utilizing legacy MEK inhibitors as templates for a reiterative structural based design and synthesis of novel, type III NCKIs (non-classical kinase inhibitors) is described. Evidence is provided that the MEK-pocket or pockets closely related to it may exist in kinases other than MEK.
Organizational Affiliation:
Pfizer Global Research and Development, Research Technology Center, Cambridge, MA 02139, USA. haile.tecle@pfizer.com