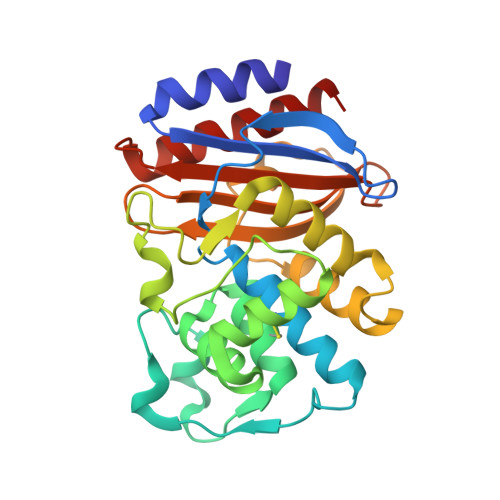
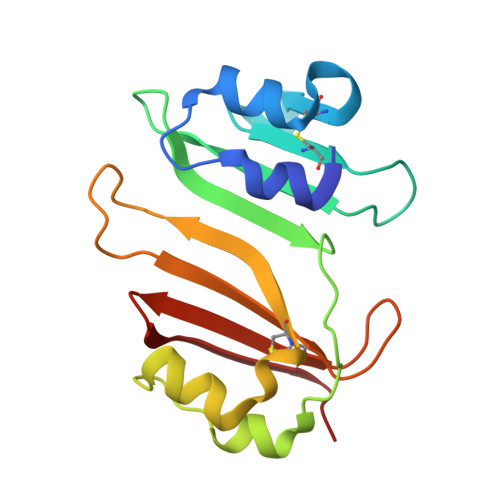
Insights into positive and negative requirements for protein-protein interactions by crystallographic analysis of the beta-lactamase inhibitory proteins BLIP, BLIP-I, and BLP.
Gretes, M., Lim, D.C., de Castro, L., Jensen, S.E., Kang, S.G., Lee, K.J., Strynadka, N.C.(2009) J Mol Biol 389: 289-305
- PubMed: 19332077
- DOI: https://doi.org/10.1016/j.jmb.2009.03.058
- Primary Citation of Related Structures:
3GMU, 3GMV, 3GMW, 3GMX, 3GMY - PubMed Abstract:
Beta-lactamase inhibitory protein (BLIP) binds a variety of beta-lactamase enzymes with wide-ranging specificity. Its binding mechanism and interface interactions are a well-established model system for the characterization of protein-protein interactions. Published studies have examined the binding of BLIP to diverse target beta-lactamases (e.g., TEM-1, SME-1, and SHV-1). However, apart from point mutations of amino acid residues, variability on the inhibitor side of this enzyme-inhibitor interface has remained unexplored. Thus, we present crystal structures of two likely BLIP relatives: (1) BLIP-I (solved alone and in complex with TEM-1), which has beta-lactamase inhibitory activity very similar to that of BLIP; and (2) beta-lactamase-inhibitory-protein-like protein (BLP) (in two apo forms, including an ultra-high-resolution structure), which is unable to inhibit any tested beta-lactamase. Despite categorical differences in species of origin and function, BLIP-I and BLP share nearly identical backbone conformations, even at loop regions differing in BLIP. We describe interacting residues and provide a comparative structural analysis of the interactions formed at the interface of BLIP-I.TEM-1 versus those formed at the interface of BLIP.TEM-1. Along with initial attempts to functionally characterize BLP, we examine its amino acid residues that structurally correspond to BLIP/BLIP-I binding hotspots to explain its inability to bind and inhibit TEM-1. We conclude that the BLIP family fold is a robust and flexible scaffold that permits the formation of high-affinity protein-protein interactions while remaining highly selective. Comparison of the two naturally occurring, distinct binding interfaces built upon this scaffold (BLIP and BLIP-I) shows that there is substantial variation possible in the subnanomolar binding interaction with TEM-1. The corresponding (non-TEM-1-binding) BLP surface shows that numerous favorable backbone-backbone/backbone-side-chain interactions with a protein partner can be negated by the presence of a few, strongly unfavorable interactions, especially electrostatic repulsions.
Organizational Affiliation:
Department of Biochemistry and Molecular Biology, Center for Blood Research, University of British Columbia, Vancouver, British Columbia, Canada.