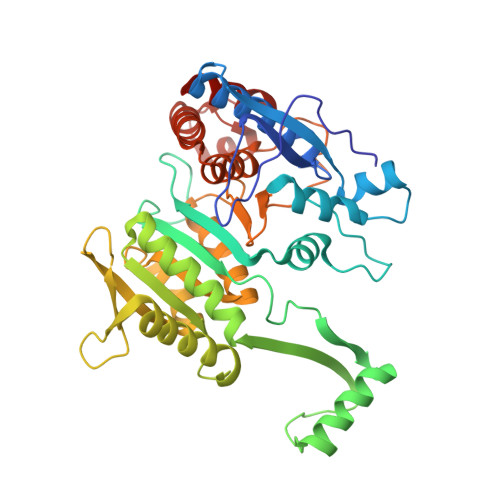
Structure of a bacterial enzyme regulated by phosphorylation, isocitrate dehydrogenase.
Hurley, J.H., Thorsness, P.E., Ramalingam, V., Helmers, N.H., Koshland Jr., D.E., Stroud, R.M.(1989) Proc Natl Acad Sci U S A 86: 8635-8639
- PubMed: 2682654
- DOI: https://doi.org/10.1073/pnas.86.22.8635
- Primary Citation of Related Structures:
3ICD - PubMed Abstract:
The structure of isocitrate dehydrogenase [threo-DS-isocitrate: NADP+ oxidoreductase (decarboxylating), EC 1.1.1.42] from Escherichia coli has been solved and refined at 2.5 A resolution and is topologically different from that of any other dehydrogenase. This enzyme, a dimer of identical 416-residue subunits, is inactivated by phosphorylation at Ser-113, which lies at the edge of an interdomain pocket that also contains many residues conserved between isocitrate dehydrogenase and isopropylmalate dehydrogenase. Isocitrate dehydrogenase contains an unusual clasp-like domain in which both polypeptide chains in the dimer interlock. Based on the structure of isocitrate dehydrogenase and conservation with isopropylmalate dehydrogenase, we suggest that the active site lies in an interdomain pocket close to the phosphorylation site.
- Department of Biochemistry and Biophysics, University of California, San Francisco 94143-0448.
Organizational Affiliation: