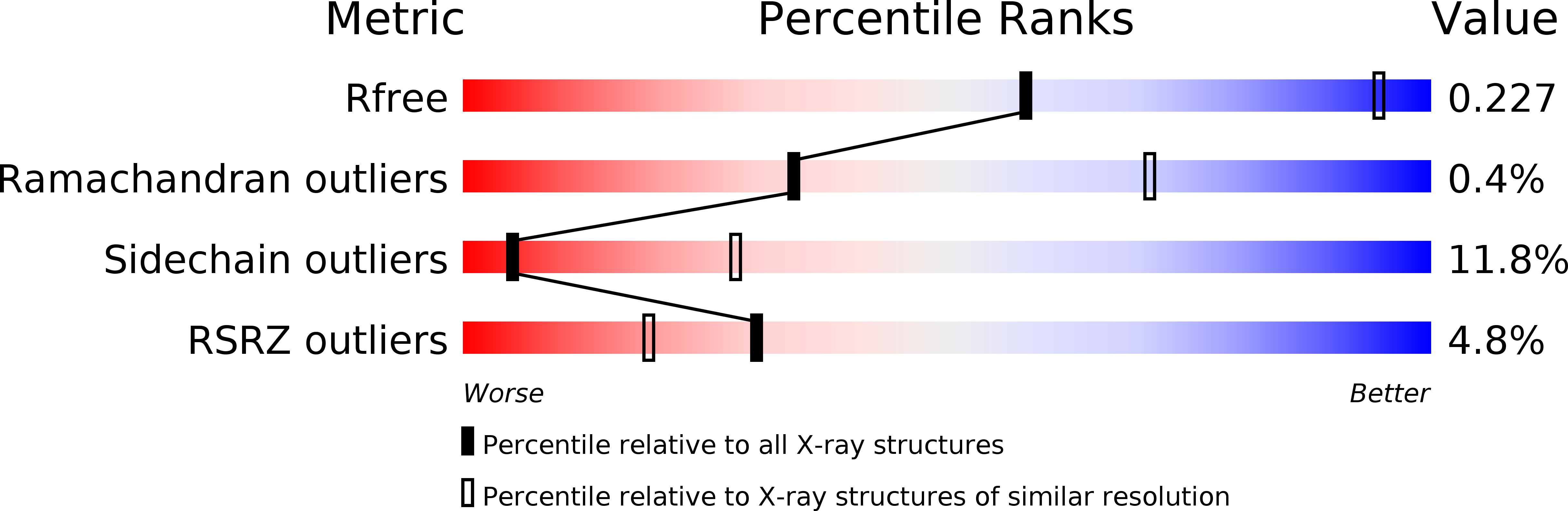
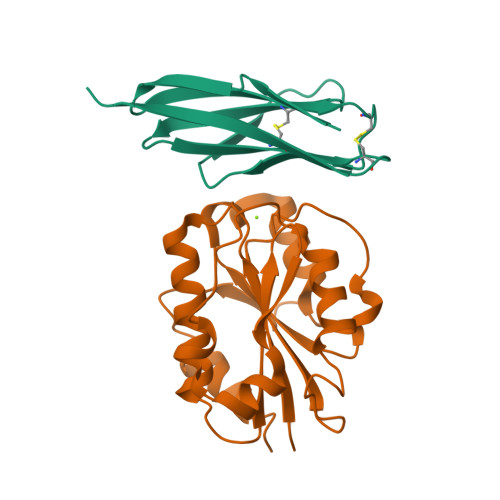
Structure of Engineered Single Domain ICAM-1 D1 with High-Affinity L Integrin I Domain of Native C-Terminal Helix Conformation
Kang, S., Kim, C.U., Gu, X., Owens, R.M., van Rijn, S.J., Boonyaleepun, V., Mao, Y., Springer, T.A., Jin, M.M.To be published.