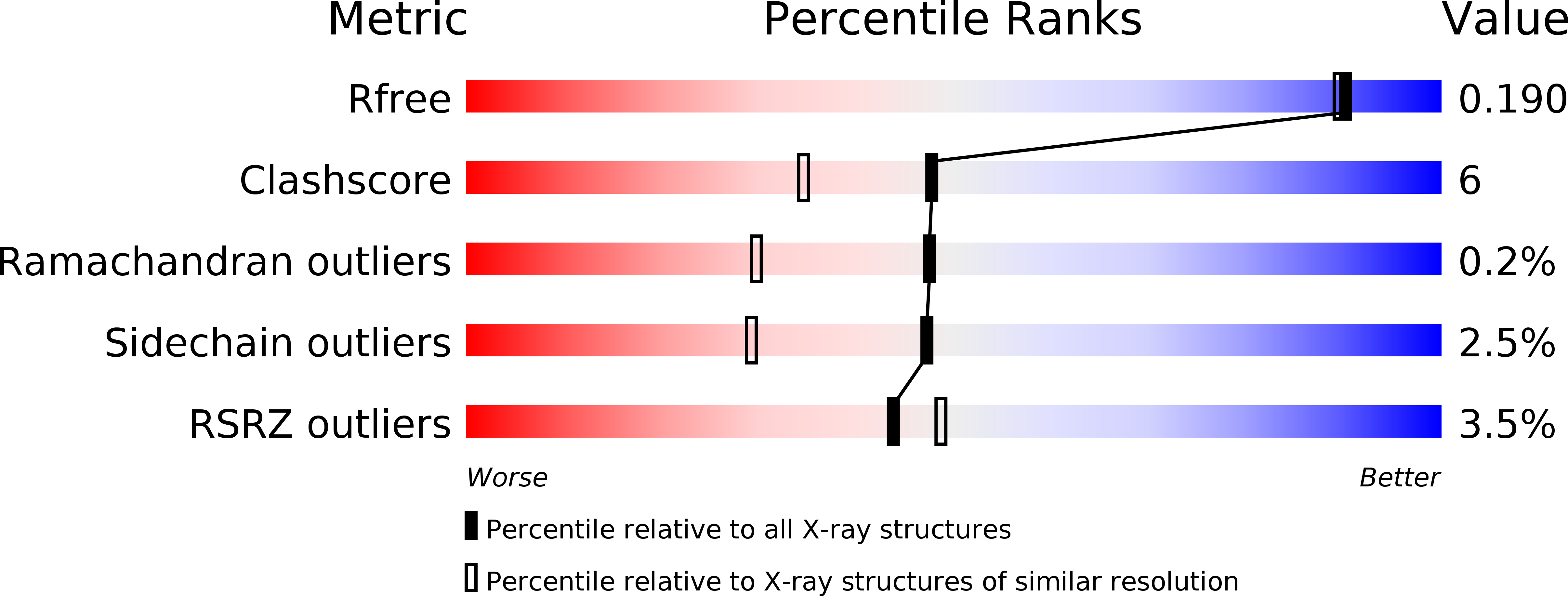
Structure of EvaA: A Paradigm for Sugar 2,3-Dehydratases.
Kubiak, R.L., Thoden, J.B., Holden, H.M.(2013) Biochemistry 52: 2078-2088
- PubMed: 23473392
- DOI: https://doi.org/10.1021/bi400176n
- Primary Citation of Related Structures:
4J7G, 4J7H - PubMed Abstract:
Unusual deoxysugars found appended to natural products often provide or enhance the pharmacokinetic activities of the parent compound. The preferred carbohydrate donors for the biosynthesis of such glycosylated natural products are the dTDP-linked sugars. Many of the biologically relevant dTDP-deoxysugars are constructed around the 2,6-dideoxyhexoses or the 2,3(4),6-trideoxyhexoses. A key step in the biosynthesis of these sugars is the removal of the hexose C-2' hydroxyl group and the oxidation of the C-3' hydroxyl group to a carbonyl moiety. Enzymes that catalyze these reactions are referred to as 2,3-dehydratases and have been, for the most part, largely uncharacterized. Here we report the first structural analysis of a sugar 2,3-dehydratase. For this investigation, the enzyme, EvaA, was cloned from Amycolatopsis orientalis, and the structure was solved and refined to a nominal resolution of 1.7 Å. On the basis of the resulting model, it is clear that EvaA belongs to the large Nudix hydrolase superfamily and is most similar to GDP-mannose hydrolase. Each subunit of the EvaA dimer folds into two domains that clearly arose via gene duplication. Two dTDP-sugar binding pockets, A and B, are present in each EvaA subunit. On the basis of site-directed mutagenesis experiments and activity assays, it appears that pocket A functions as the active site and pocket B is simply a remnant left behind from the gene duplication event. As 2,3-dehydration is crucial for the biosynthesis of many unusual deoxysugars, this investigation provides key structural insight into this widely conserved reaction.
Organizational Affiliation:
Department of Biochemistry, University of Wisconsin , Madison, Wisconsin 53706, United States.