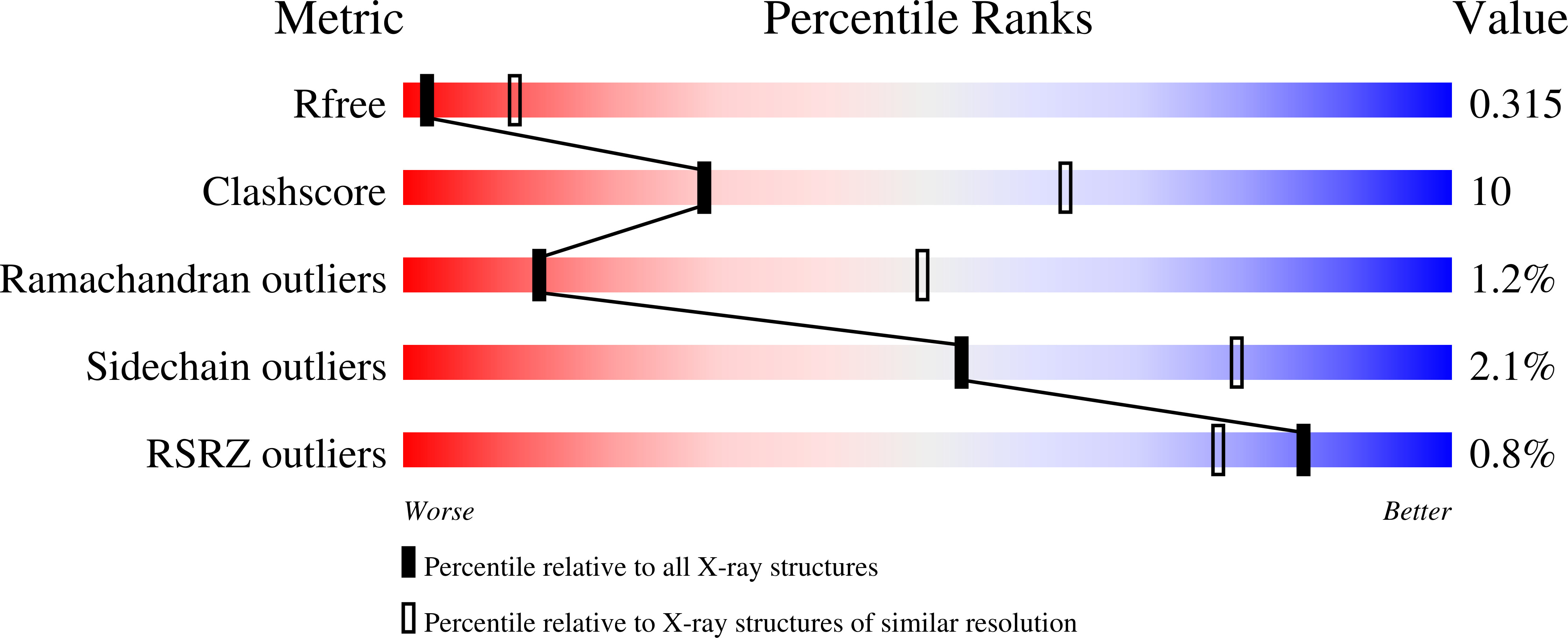
Computational design of ligand-binding proteins with high affinity and selectivity.
Tinberg, C.E., Khare, S.D., Dou, J., Doyle, L., Nelson, J.W., Schena, A., Jankowski, W., Kalodimos, C.G., Johnsson, K., Stoddard, B.L., Baker, D.(2013) Nature 501: 212-216
- PubMed: 24005320
- DOI: https://doi.org/10.1038/nature12443
- Primary Citation of Related Structures:
4J8T, 4J9A - PubMed Abstract:
The ability to design proteins with high affinity and selectivity for any given small molecule is a rigorous test of our understanding of the physiochemical principles that govern molecular recognition. Attempts to rationally design ligand-binding proteins have met with little success, however, and the computational design of protein-small-molecule interfaces remains an unsolved problem. Current approaches for designing ligand-binding proteins for medical and biotechnological uses rely on raising antibodies against a target antigen in immunized animals and/or performing laboratory-directed evolution of proteins with an existing low affinity for the desired ligand, neither of which allows complete control over the interactions involved in binding. Here we describe a general computational method for designing pre-organized and shape complementary small-molecule-binding sites, and use it to generate protein binders to the steroid digoxigenin (DIG). Of seventeen experimentally characterized designs, two bind DIG; the model of the higher affinity binder has the most energetically favourable and pre-organized interface in the design set. A comprehensive binding-fitness landscape of this design, generated by library selections and deep sequencing, was used to optimize its binding affinity to a picomolar level, and X-ray co-crystal structures of two variants show atomic-level agreement with the corresponding computational models. The optimized binder is selective for DIG over the related steroids digitoxigenin, progesterone and β-oestradiol, and this steroid binding preference can be reprogrammed by manipulation of explicitly designed hydrogen-bonding interactions. The computational design method presented here should enable the development of a new generation of biosensors, therapeutics and diagnostics.
Organizational Affiliation:
Department of Biochemistry, University of Washington, Seattle, WA 98195, USA.