Crystal structure of a phosphorylation-coupled vitamin C transporter.
Luo, P., Yu, X., Wang, W., Fan, S., Li, X., Wang, J.(2015) Nat Struct Mol Biol 22: 238-241
- PubMed: 25686089
- DOI: https://doi.org/10.1038/nsmb.2975
- Primary Citation of Related Structures:
4RP8, 4RP9 - PubMed Abstract:
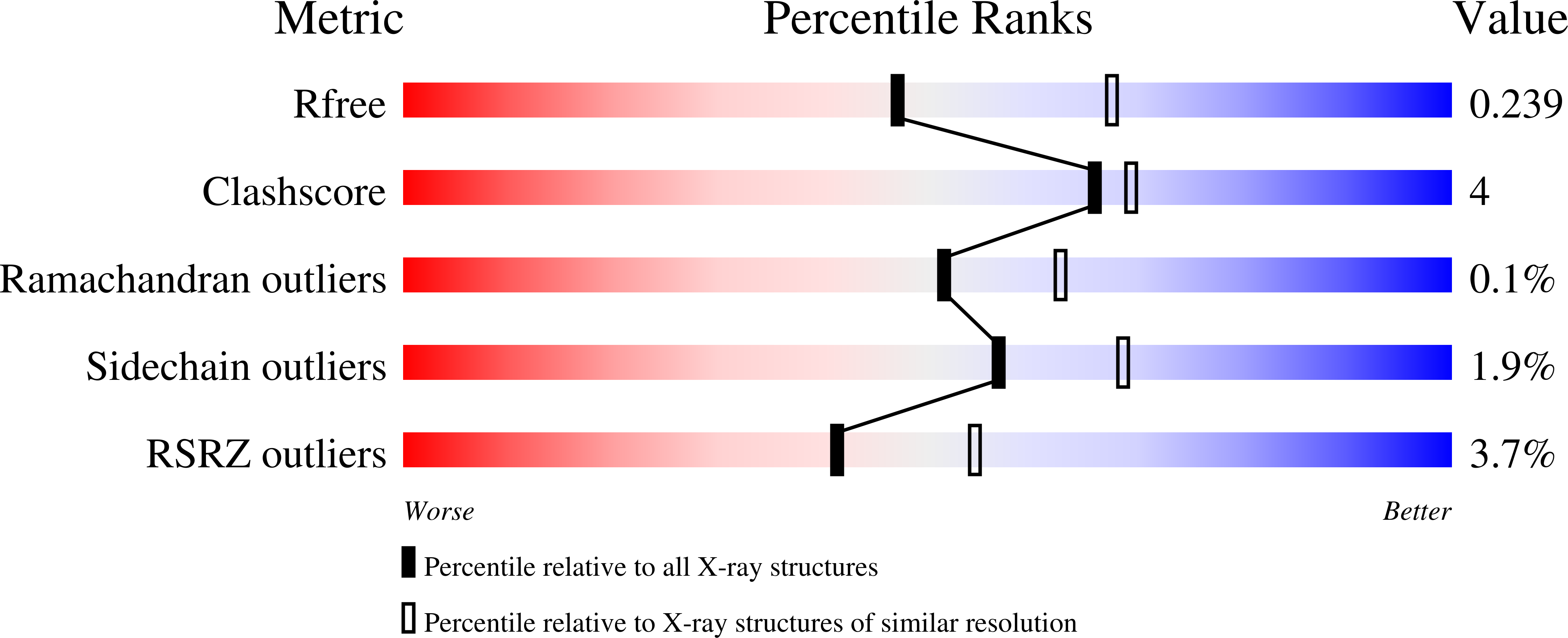
Bacteria use vitamin C (L-ascorbic acid) as a carbon source under anaerobic conditions. The phosphoenolpyruvate-dependent phosphotransferase system (PTS), comprising a transporter (UlaA), a IIB-like enzyme (UlaB) and a IIA-like enzyme (UlaC), is required for the anaerobic uptake of vitamin C and its phosphorylation to L-ascorbate 6-phosphate. Here, we present the crystal structures of vitamin C-bound UlaA from Escherichia coli in two conformations at 1.65-Å and 2.35-Å resolution. UlaA forms a homodimer and exhibits a new fold. Each UlaA protomer consists of 11 transmembrane segments arranged into a 'V-motif' domain and a 'core' domain. The V motifs form the interface between the two protomers, and the core-domain residues coordinate vitamin C. The alternating access of the substrate from the opposite side of the cell membrane may be achieved through rigid-body rotation of the core relative to the V motif.
Organizational Affiliation:
1] State Key Laboratory of Biomembrane and Membrane Biotechnology, School of Life Sciences, Tsinghua University, Beijing, China. [2] Center for Structural Biology, School of Life Sciences, Tsinghua University, Beijing, China.