Structural Characterization of the N-Terminal Part of the Mers-Cov Nucleocapsid by X-Ray Diffraction and Small-Angle X-Ray Scattering
Papageorgiou, N., Lichiere, J., Baklouti, A., Ferron, F., Canard, B., Coutard, B.(2016) Acta Crystallogr D Biol Crystallogr 72: 192
- PubMed: 26894667
- DOI: https://doi.org/10.1107/S2059798315024328
- Primary Citation of Related Structures:
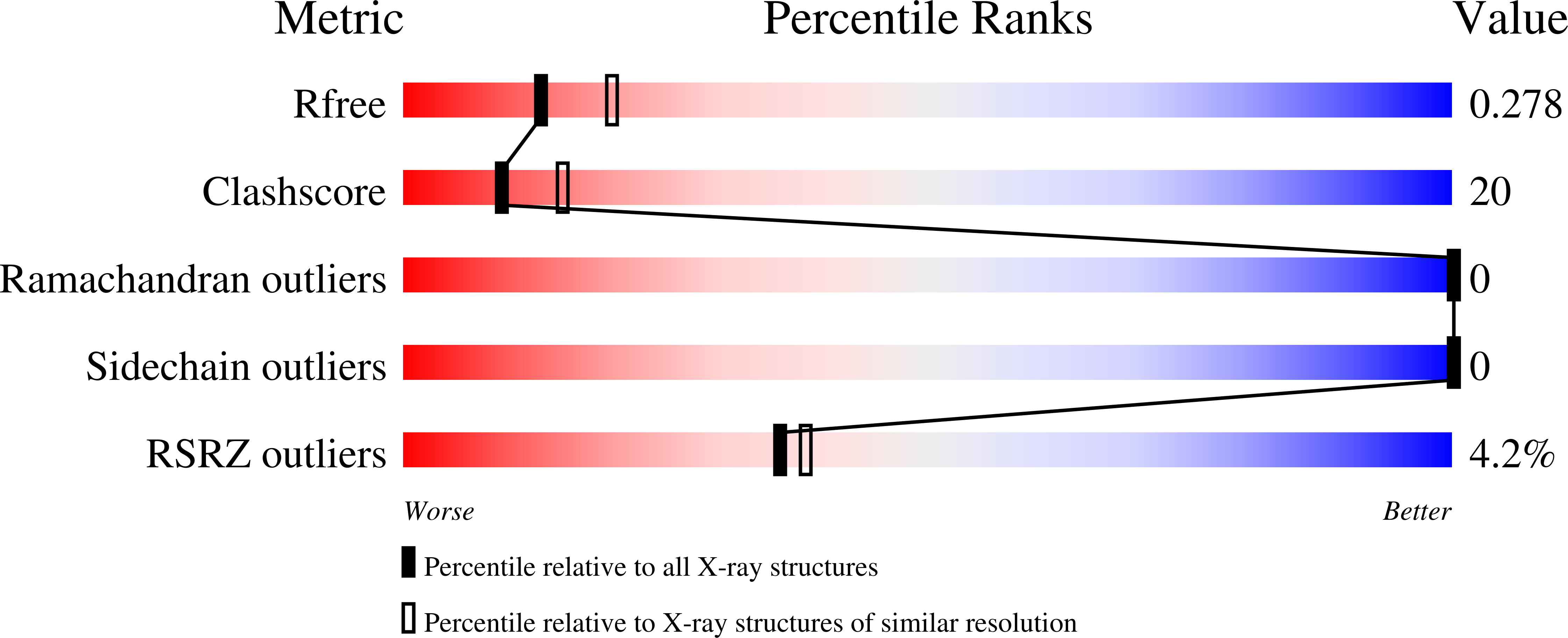
4UD1 - PubMed Abstract:
The N protein of coronaviruses is a multifunctional protein that is organized into several domains. The N-terminal part is composed of an intrinsically disordered region (IDR) followed by a structured domain called the N-terminal domain (NTD). In this study, the structure determination of the N-terminal region of the MERS-CoV N protein via X-ray diffraction measurements is reported at a resolution of 2.4 Å. Since the first 30 amino acids were not resolved by X-ray diffraction, the structural study was completed by a SAXS experiment to propose a structural model including the IDR. This model presents the N-terminal region of the MERS-CoV as a monomer that displays structural features in common with other coronavirus NTDs.
Organizational Affiliation:
CNRS, AFMB UMR 7257, 13288 Marseille, France.