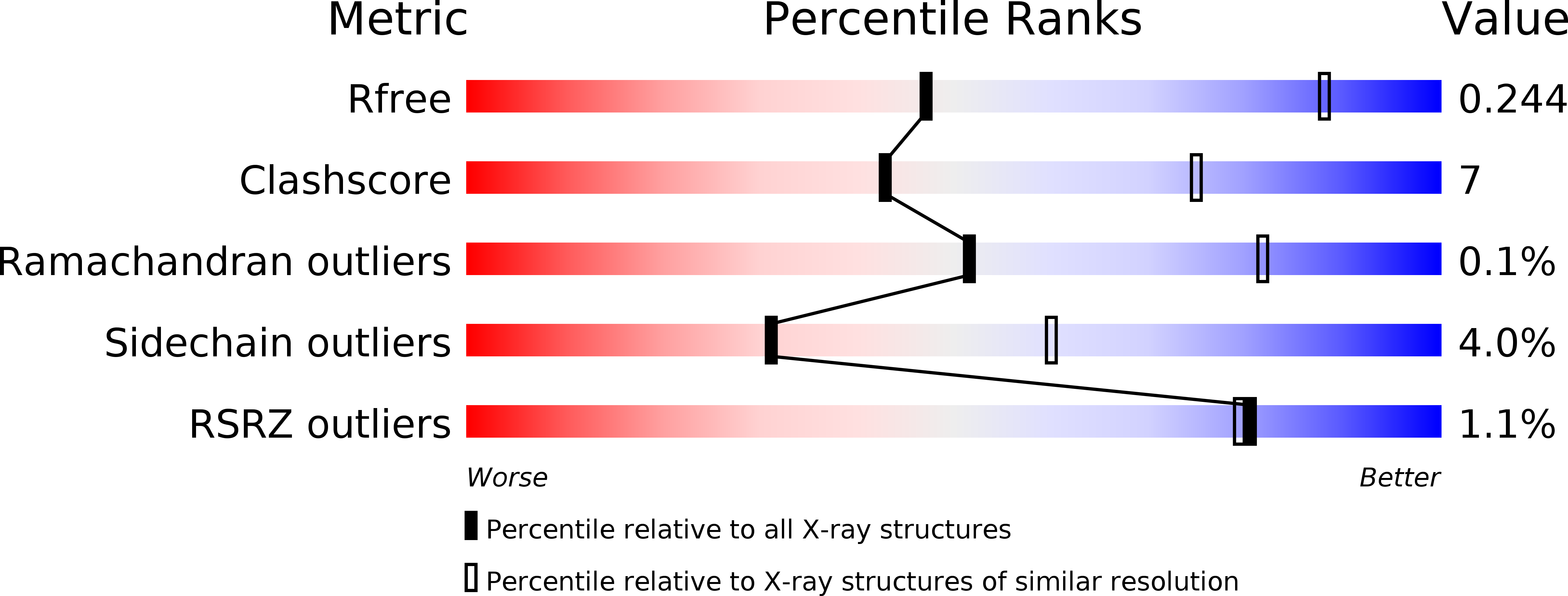
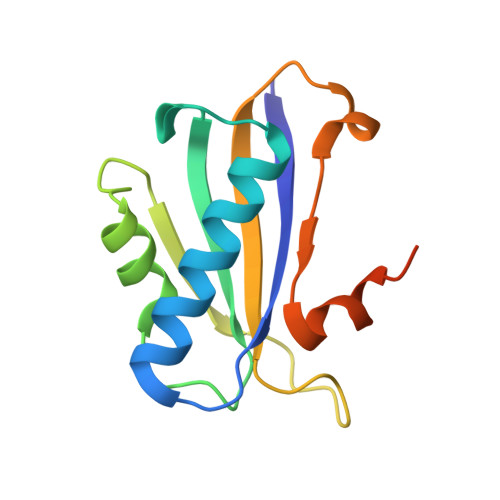
Structure of a designed tetrahedral protein assembly variant engineered to have improved soluble expression.
Bale, J.B., Park, R.U., Liu, Y., Gonen, S., Gonen, T., Cascio, D., King, N.P., Yeates, T.O., Baker, D.(2015) Protein Sci 24: 1695-1701
- PubMed: 26174163
- DOI: https://doi.org/10.1002/pro.2748
- Primary Citation of Related Structures:
4ZK7 - PubMed Abstract:
We recently reported the development of a computational method for the design of coassembling multicomponent protein nanomaterials. While four such materials were validated at high-resolution by X-ray crystallography, low yield of soluble protein prevented X-ray structure determination of a fifth designed material, T33-09. Here we report the design and crystal structure of T33-31, a variant of T33-09 with improved soluble yield resulting from redesign efforts focused on mutating solvent-exposed side chains to charged amino acids. The structure is found to match the computational design model with atomic-level accuracy, providing further validation of the design approach and demonstrating a simple and potentially general means of improving the yield of designed protein nanomaterials.
Organizational Affiliation:
Department of Biochemistry, University of Washington, Seattle, Washington, 98195.