Through-bond effects in the ternary complexes of thrombin sandwiched by two DNA aptamers.
Pica, A., Russo Krauss, I., Parente, V., Tateishi-Karimata, H., Nagatoishi, S., Tsumoto, K., Sugimoto, N., Sica, F.(2017) Nucleic Acids Res 45: 461-469
- PubMed: 27899589
- DOI: https://doi.org/10.1093/nar/gkw1113
- Primary Citation of Related Structures:
5EW1, 5EW2 - PubMed Abstract:
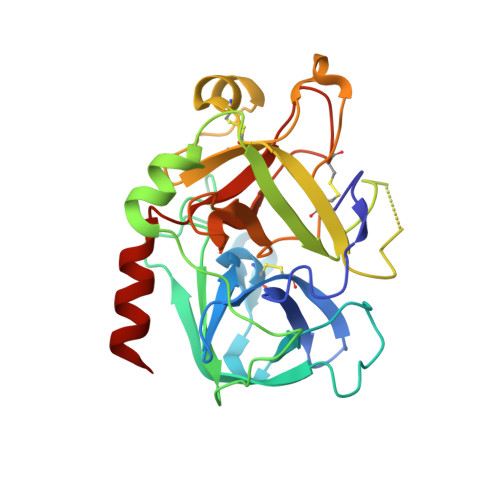
Aptamers directed against human thrombin can selectively bind to two different exosites on the protein surface. The simultaneous use of two DNA aptamers, HD1 and HD22, directed to exosite I and exosite II respectively, is a very powerful approach to exploit their combined affinity. Indeed, strategies to link HD1 and HD22 together have been proposed in order to create a single bivalent molecule with an enhanced ability to control thrombin activity. In this work, the crystal structures of two ternary complexes, in which thrombin is sandwiched between two DNA aptamers, are presented and discussed. The structures shed light on the cross talk between the two exosites. The through-bond effects are particularly evident at exosite II, with net consequences on the HD22 structure. Moreover, thermodynamic data on the binding of the two aptamers are also reported and analyzed.
Organizational Affiliation:
Department of Chemical Sciences, University of Naples Federico II, Via Cintia, I-80126 Naples, Italy.