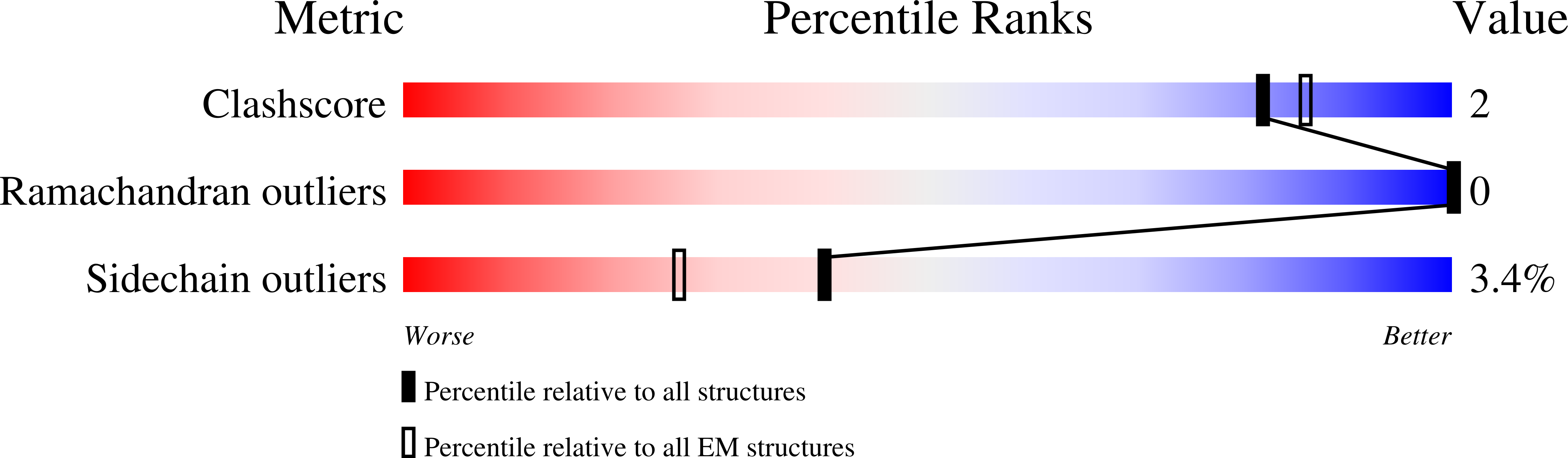A Molecular-Level Account of the Antigenic Hantaviral Surface.
Li, S., Rissanen, I., Zeltina, A., Hepojoki, J., Raghwani, J., Harlos, K., Pybus, O.G., Huiskonen, J.T., Bowden, T.A.(2016) Cell Rep 15: 959
- PubMed: 27117403
- DOI: https://doi.org/10.1016/j.celrep.2016.03.082
- Primary Citation of Related Structures:
5FXU, 5FYN - PubMed Abstract:
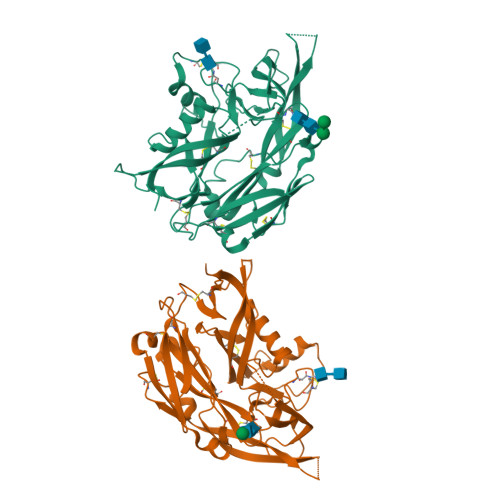
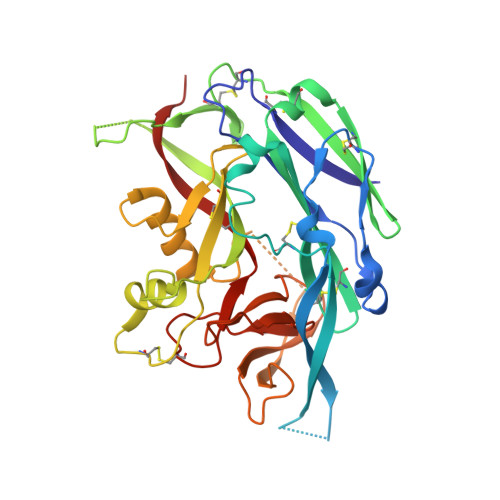
Hantaviruses, a geographically diverse group of zoonotic pathogens, initiate cell infection through the concerted action of Gn and Gc viral surface glycoproteins. Here, we describe the high-resolution crystal structure of the antigenic ectodomain of Gn from Puumala hantavirus (PUUV), a causative agent of hemorrhagic fever with renal syndrome. Fitting of PUUV Gn into an electron cryomicroscopy reconstruction of intact Gn-Gc spike complexes from the closely related but non-pathogenic Tula hantavirus localized Gn tetramers to the membrane-distal surface of the virion. The accuracy of the fitting was corroborated by epitope mapping and genetic analysis of available PUUV sequences. Interestingly, Gn exhibits greater non-synonymous sequence diversity than the less accessible Gc, supporting a role of the host humoral immune response in exerting selective pressure on the virus surface. The fold of PUUV Gn is likely to be widely conserved across hantaviruses.
Organizational Affiliation:
Division of Structural Biology, Wellcome Trust Centre for Human Genetics, University of Oxford, Roosevelt Drive, Oxford OX3 7BN, UK.