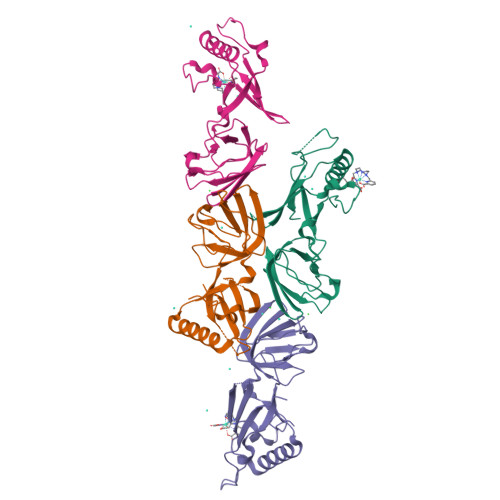
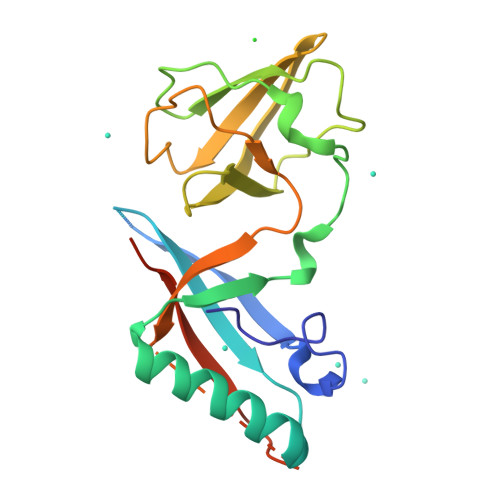
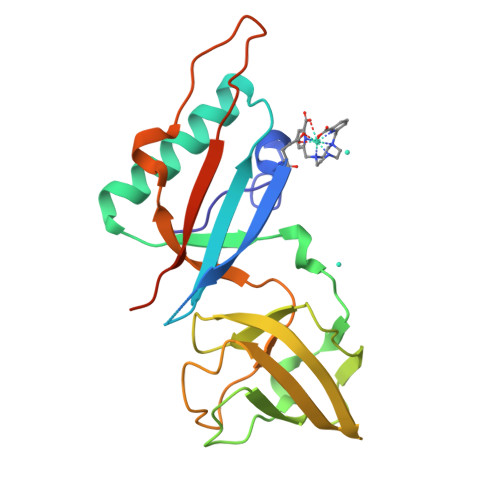
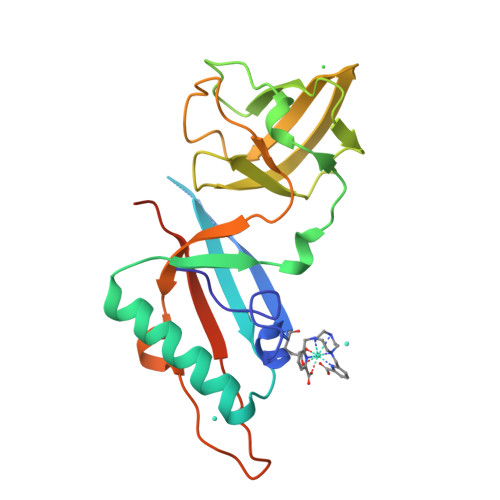
Crystallophore: a versatile lanthanide complex for protein crystallography combining nucleating effects, phasing properties, and luminescence.
Engilberge, S., Riobe, F., Di Pietro, S., Lassalle, L., Coquelle, N., Arnaud, C.A., Pitrat, D., Mulatier, J.C., Madern, D., Breyton, C., Maury, O., Girard, E.(2017) Chem Sci 8: 5909-5917
- PubMed: 29619195
- DOI: https://doi.org/10.1039/c7sc00758b
- Primary Citation of Related Structures:
5MF2 - PubMed Abstract:
Macromolecular crystallography suffers from two major issues: getting well-diffracting crystals and solving the phase problem inherent to large macromolecules. Here, we describe the first example of a lanthanide complex family named "crystallophore" (Xo4), which contributes to tackling both bottlenecks. This terbium complex, Tb-Xo4, is an appealing agent for biocrystallography, combining the exceptional phasing power of the Tb(iii) heavy atom with powerful nucleating properties, providing ready-to-use crystals for structure determination. Furthermore, protein/Tb-Xo4 co-crystals can be easily detected and discriminated from other crystalline by-products using luminescence. We demonstrate the potential of this additive for the crystallisation and structure determination of eight proteins, two of whose structures were unknown.
Organizational Affiliation:
Univ. Grenoble Alpes , CEA , CNRS , IBS , F-38000 Grenoble , France . Email: eric.girard@ibs.fr.