Structural Insights into Subunits Assembly and the Oxyester Splicing Mechanism of Neq pol Split Intein.
Gordo, V., Aparicio, D., Perez-Luque, R., Benito, A., Vilanova, M., Uson, I., Fita, I., Ribo, M.(2018) Cell Chem Biol 25: 871-879.e2
- PubMed: 29754955
- DOI: https://doi.org/10.1016/j.chembiol.2018.04.008
- Primary Citation of Related Structures:
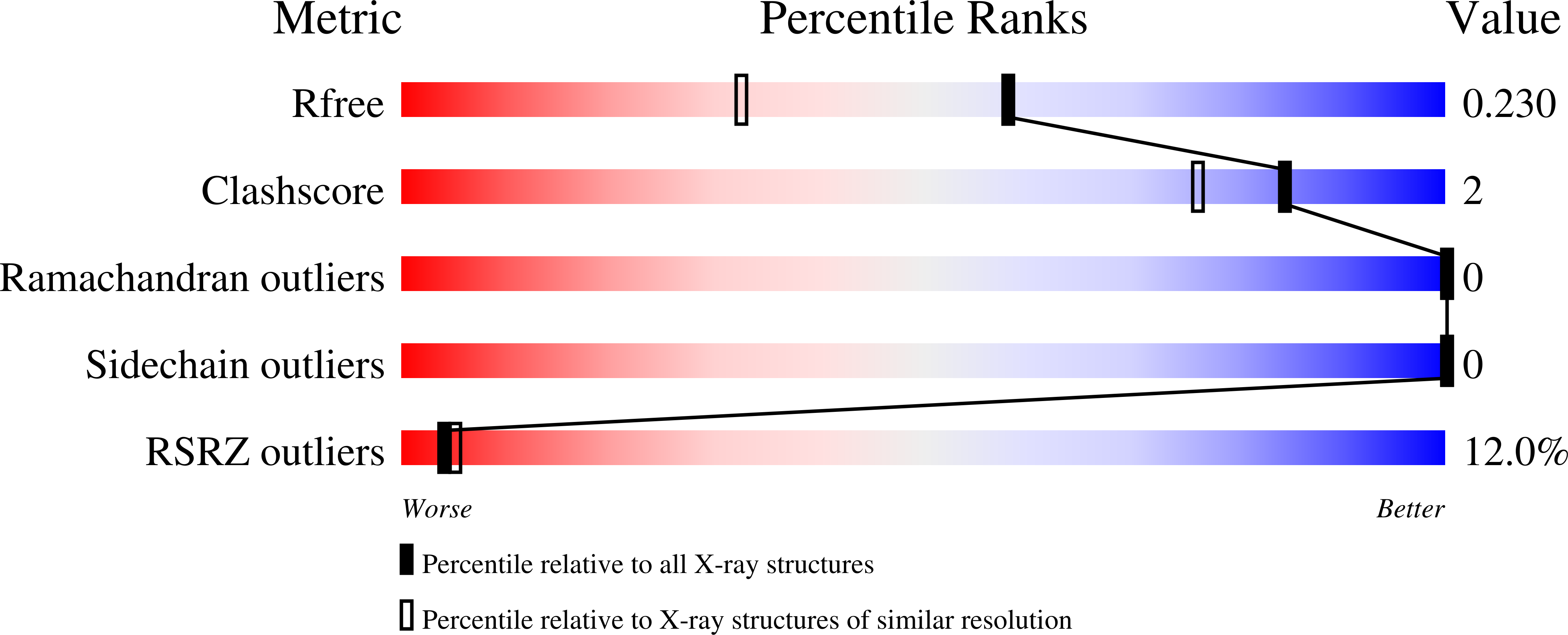
5OXW, 5OXX, 5OXZ - PubMed Abstract:
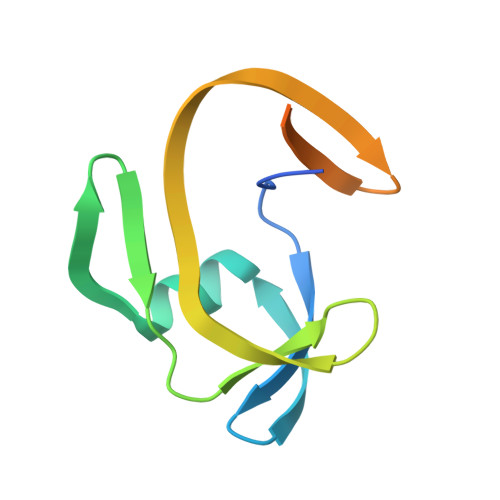
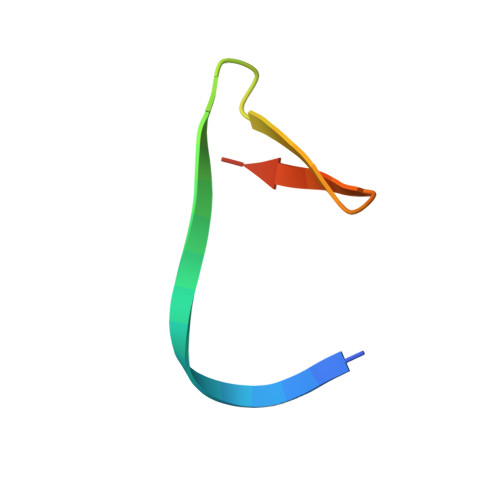
Split inteins are expressed as two separated subunits (N-intein and C-intein) fused to the corresponding exteins. The specific association of both intein subunits precedes protein splicing, which results in excision of the intein subunits and in ligation, by a peptide bond, of the concomitant exteins. Catalytically active intein precursors are typically too reactive for crystallization or even isolation. Neq pol is the trans-intein of the B-type DNA polymerase I split gene from hyperthermophile Nanoarchaeum equitans. We have determined the crystal structures of both the isolated NeqN and the complex of NeqN and NeqC subunits carrying the wild-type sequences, including the essential catalytic residues Ser1 and Thr+1, in addition to seven and three residues of the N- and C-exteins, respectively. These structures provide detailed information on the unique oxyester chemistry of the splicing mechanism of Neq pol and of the extensive rearrangements that occur in NeqN during the association step.
Organizational Affiliation:
Laboratori d'Enginyeria de Proteïnes, Departament de Biologia, Facultat de Ciències, Universitat de Girona, C/ Maria Aurèlia Capmany 40, 17003 Girona, Spain; IdIBGi Hospital Universitari Josep Trueta, Girona, Spain.