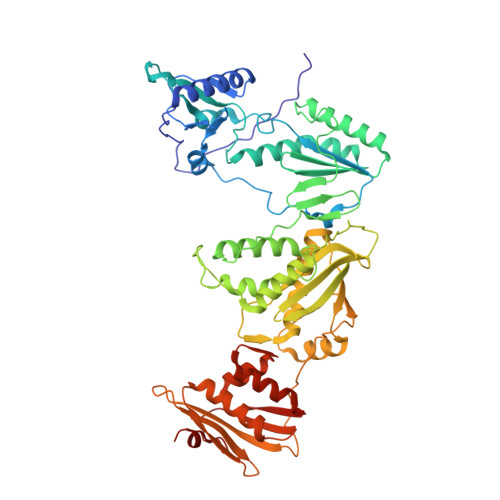
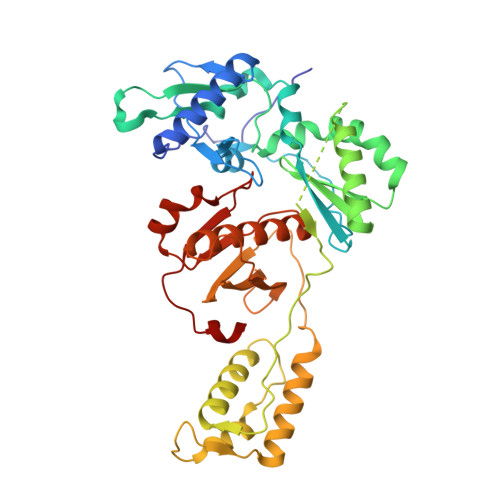
Design, Conformation, and Crystallography of 2-Naphthyl Phenyl Ethers as Potent Anti-HIV Agents.
Lee, W.G., Chan, A.H., Spasov, K.A., Anderson, K.S., Jorgensen, W.L.(2016) ACS Med Chem Lett 7: 1156-1160
- PubMed: 27994756
- DOI: https://doi.org/10.1021/acsmedchemlett.6b00390
- Primary Citation of Related Structures:
5TER - PubMed Abstract:
Catechol diethers that incorporate a 7-cyano-2-naphthyl substituent are reported as non-nucleoside inhibitors of HIV-1 reverse transcriptase (NNRTIs). Many of the compounds have 1-10 nM potencies toward wild-type HIV-1. An interesting conformational effect allows two unique conformers for the naphthyl group in complexes with HIV-RT. X-ray crystal structures for 4a and 4f illustrate the alternatives.
- Department of Chemistry, Yale University , New Haven, Connecticut 06520-8107, United States.
Organizational Affiliation: