Structural basis of transcription arrest by coliphage HK022 Nun in anEscherichia coliRNA polymerase elongation complex.
Kang, J.Y., Olinares, P.D., Chen, J., Campbell, E.A., Mustaev, A., Chait, B.T., Gottesman, M.E., Darst, S.A.(2017) Elife 6
- PubMed: 28318486
- DOI: https://doi.org/10.7554/eLife.25478
- Primary Citation of Related Structures:
6ALF, 6ALG, 6ALH - PubMed Abstract:
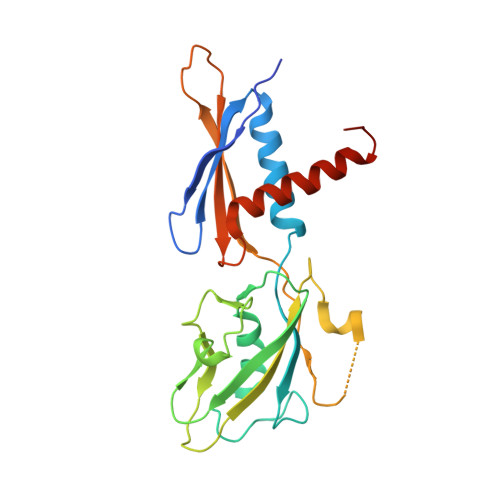
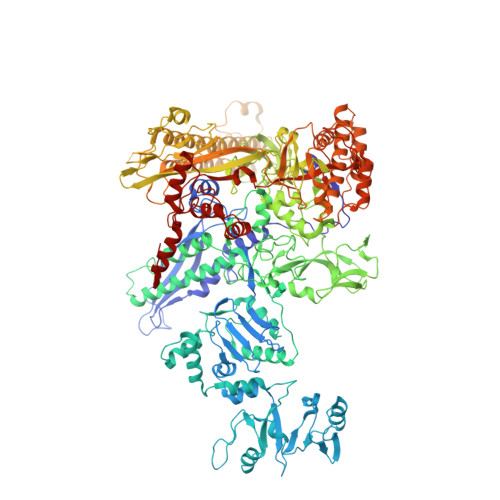
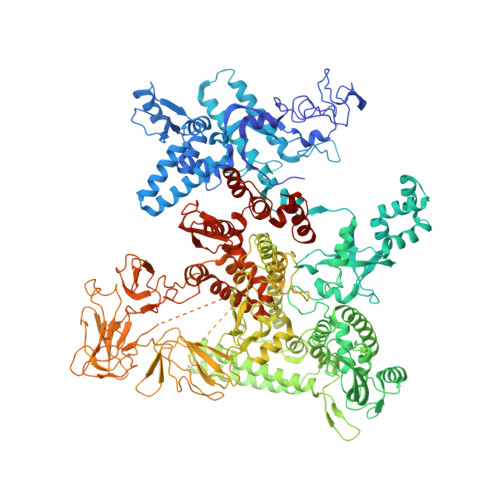
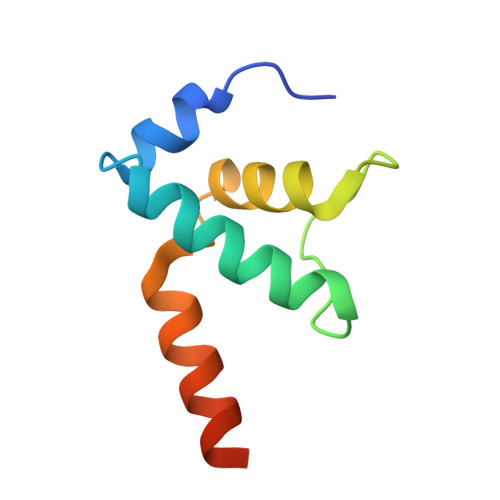
Coliphage HK022 Nun blocks superinfection by coliphage λ by stalling RNA polymerase (RNAP) translocation specifically on λ DNA. To provide a structural framework to understand how Nun blocks RNAP translocation, we determined structures of Escherichia coli RNAP ternary elongation complexes (TECs) with and without Nun by single-particle cryo-electron microscopy. Nun fits tightly into the TEC by taking advantage of gaps between the RNAP and the nucleic acids. The C-terminal segment of Nun interacts with the RNAP β and β' subunits inside the RNAP active site cleft as well as with nearly every element of the nucleic acid scaffold, essentially crosslinking the RNAP and the nucleic acids to prevent translocation, a mechanism supported by the effects of Nun amino acid substitutions. The nature of Nun interactions inside the RNAP active site cleft suggests that RNAP clamp opening is required for Nun to establish its interactions, explaining why Nun acts on paused TECs.
- Laboratory of Molecular Biophysics, The Rockefeller University, New York City, United States.
Organizational Affiliation: