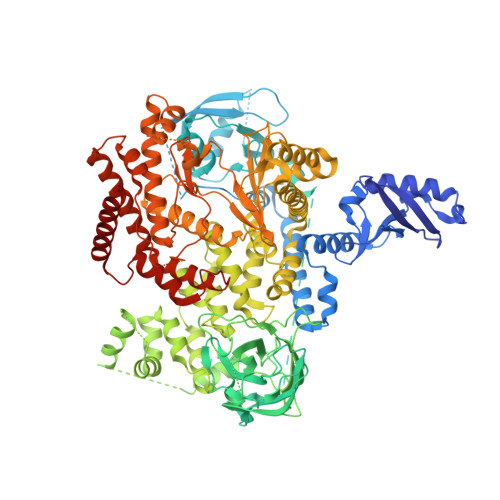
Discovery and optimization of heteroaryl piperazines as potent and selective PI3K delta inhibitors.
Zhou, H., McGowan, M.A., Lipford, K., Christopher, M., Fradera, X., Witter, D., Lesburg, C.A., Li, C., Methot, J.L., Lampe, J., Achab, A., Shaffer, L., Goldenblatt, P., Shah, S., Bass, A., Schroeder, G., Chen, D., Zeng, H., Augustin, M.A., Katz, J.D.(2020) Bioorg Med Chem Lett 30: 126715-126715
- PubMed: 31757666
- DOI: https://doi.org/10.1016/j.bmcl.2019.126715
- Primary Citation of Related Structures:
6OCO, 6OCU - PubMed Abstract:
A high-throughput screening (HTS) campaign identified a class of heteroaryl piperazines with excellent baseline affinity and selectivity for phosphoinositide 3-kinase δ (PI3Kδ) over closely related isoforms. Rapid evaluation and optimization of structure-activity relationships (SAR) for this class, leveraging the modular nature of this scaffold, facilitated development of this hit class into a series of potent and selective inhibitors of PI3Kδ. This effort culminated in the identification of 29, which displayed excellent potency in enzyme and cell-based assays, as well as favorable pharmacokinetic and off-target profiles.
Organizational Affiliation:
Department of Discovery Chemistry, Merck & Co., Inc., 33 Avenue Louis Pasteur, Boston, MA 02115, USA. Electronic address: hua_zhou2@merck.com.