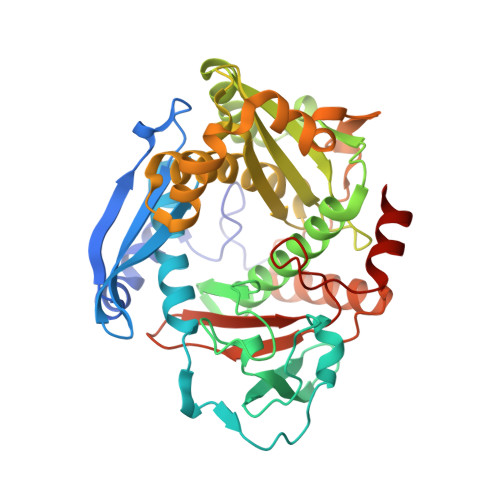
Mechanistic Basis for Ribosomal Peptide Backbone Modifications.
Dong, S.H., Liu, A., Mahanta, N., Mitchell, D.A., Nair, S.K.(2019) ACS Cent Sci 5: 842-851
- PubMed: 31139720
- DOI: https://doi.org/10.1021/acscentsci.9b00124
- Primary Citation of Related Structures:
6PE3, 6PEU - PubMed Abstract:
YcaO enzymes are known to catalyze the ATP-dependent formation of azoline heterocycles, thioamides, and (macro)lactamidines on peptide substrates. These enzymes are found in multiple biosynthetic pathways, including those for several different classes of ribosomally synthesized and post-translationally modified peptides (RiPPs). However, there are major knowledge gaps in the mechanistic and structural underpinnings that govern each of the known YcaO-mediated modifications. Here, we present the first structure of any YcaO enzyme bound to its peptide substrate in the active site, specifically that from Methanocaldococcus jannaschii which is involved in the thioamidation of the α-subunit of methyl-coenzyme M reductase (McrA). The structural data are leveraged to identify and test the residues involved in substrate binding and catalysis by site-directed mutagenesis. We also show that thioamide-forming YcaOs can carry out the cyclodehydration of a related peptide substrate, which underscores the mechanistic conservation across the YcaO family and allows for the extrapolation of mechanistic details to azoline-forming YcaOs involved in RiPP biosynthesis. A bioinformatic survey of all YcaOs highlights the diverse sequence space in azoline-forming YcaOs and suggests their early divergence from a common ancestor. The data presented within provide a detailed molecular framework for understanding this family of enzymes, which reconcile several decades of prior data on RiPP cyclodehydratases. These studies also provide the foundational knowledge to impact our mechanistic understanding of additional RiPP biosynthetic classes.
Organizational Affiliation:
Department of Biochemistry, Carl R. Woese Institute for Genomic Biology, Department of Microbiology, Department of Chemistry, and Center for Biophysics and Quantitative Biology, University of Illinois, 600 South Mathews Avenue, Urbana, Illinois 61801, United States.