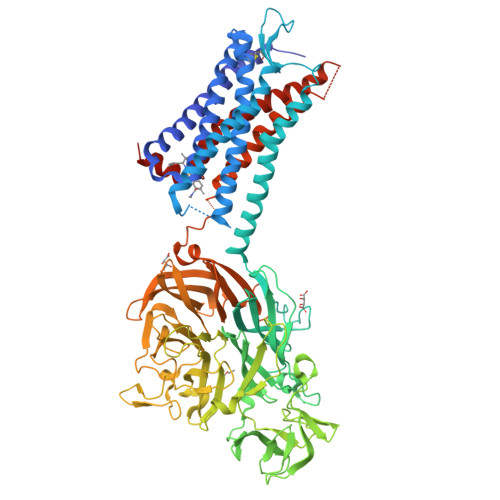
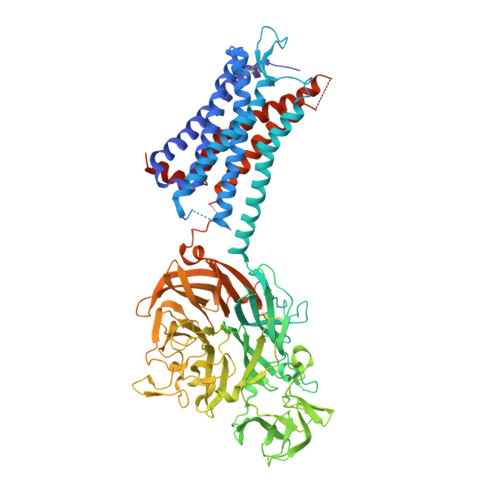
Structural Basis for Allosteric Ligand Recognition in the Human CC Chemokine Receptor 7.
Jaeger, K., Bruenle, S., Weinert, T., Guba, W., Muehle, J., Miyazaki, T., Weber, M., Furrer, A., Haenggi, N., Tetaz, T., Huang, C.Y., Mattle, D., Vonach, J.M., Gast, A., Kuglstatter, A., Rudolph, M.G., Nogly, P., Benz, J., Dawson, R.J.P., Standfuss, J.(2019) Cell 178: 1222-1230.e10
- PubMed: 31442409
- DOI: https://doi.org/10.1016/j.cell.2019.07.028
- Primary Citation of Related Structures:
6QZH - PubMed Abstract:
The CC chemokine receptor 7 (CCR7) balances immunity and tolerance by homeostatic trafficking of immune cells. In cancer, CCR7-mediated trafficking leads to lymph node metastasis, suggesting the receptor as a promising therapeutic target. Here, we present the crystal structure of human CCR7 fused to the protein Sialidase NanA by using data up to 2.1 Å resolution. The structure shows the ligand Cmp2105 bound to an intracellular allosteric binding pocket. A sulfonamide group, characteristic for various chemokine receptor ligands, binds to a patch of conserved residues in the Gi protein binding region between transmembrane helix 7 and helix 8. We demonstrate how structural data can be used in combination with a compound repository and automated thermal stability screening to identify and modulate allosteric chemokine receptor antagonists. We detect both novel (CS-1 and CS-2) and clinically relevant (CXCR1-CXCR2 phase-II antagonist Navarixin) CCR7 modulators with implications for multi-target strategies against cancer.
Organizational Affiliation:
Laboratory of Biomolecular Research, Department of Biology and Chemistry, Paul Scherrer Institute, Villigen PSI.