Mustards-Derived Terpyridine-Platinum Complexes as Anticancer Agents: DNA Alkylation vs Coordination.
Adams, M., Sullivan, M.P., Tong, K.K.H., Goldstone, D.C., Hanif, M., Jamieson, S.M.F., Hartinger, C.G.(2021) Inorg Chem 60: 2414-2424
- PubMed: 33497565
- DOI: https://doi.org/10.1021/acs.inorgchem.0c03317
- Primary Citation of Related Structures:
6WX5 - PubMed Abstract:
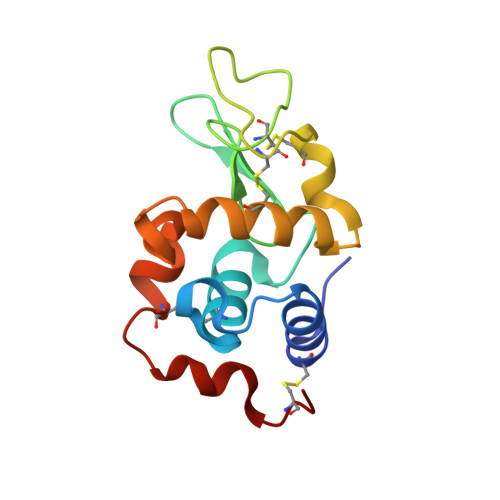
The development of bifunctional platinum complexes with the ability to interact with DNA via different binding modes is of interest in anticancer metallodrug research. Therefore, we report platinum(II) terpyridine complexes to target DNA by coordination and/or through a tethered alkylating moiety. The platinum complexes were evaluated for their in vitro antiproliferative properties against the human cancer cell lines HCT116 (colorectal), SW480 (colon), NCI-H460 (non-small cell lung), and SiHa (cervix) and generally exhibited potent antiproliferative activity although lower than their respective terpyridine ligands. 1 H NMR spectroscopy and/or ESI-MS studies on the aqueous stability and reactivity with various small biomolecules, acting as protein and DNA model compounds, were used to establish potential modes of action for these complexes. These investigations indicated rapid binding of complex PtL3 to the biomolecules through coordination to the Pt center, while PtL4 in addition alkylated 9-ethylguanine. PtL3 was investigated for its reactivity to the model protein hen egg white lysozyme (HEWL) by protein crystallography which allowed identification of the N δ1 atom of His15 as the binding site.
Organizational Affiliation:
School of Chemical Sciences, University of Auckland, Private Bag 92019, Auckland 1142, New Zealand.