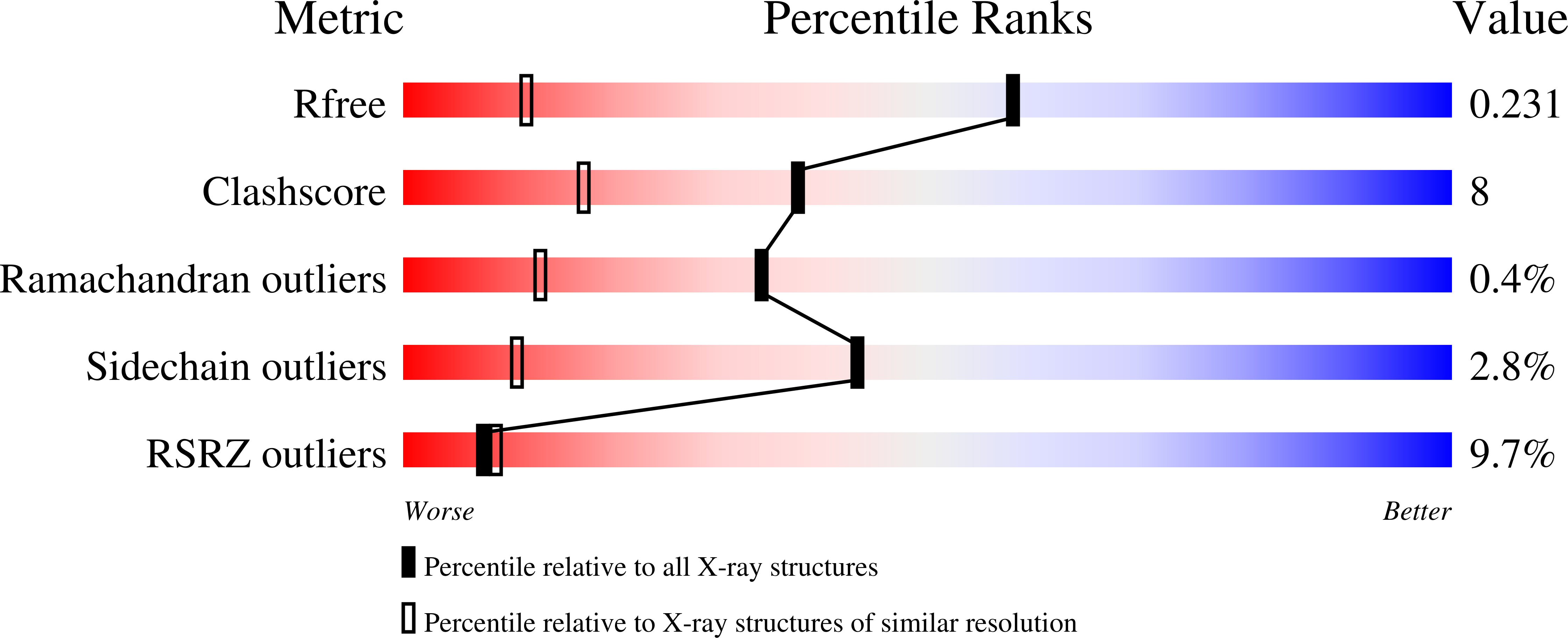
The Enzyme-Free Release of Nucleotides from Phosphoramidates Depends Strongly on the Amino Acid.
Jovanovic, D., Tremmel, P., Pallan, P.S., Egli, M., Richert, C.(2020) Angew Chem Int Ed Engl 59: 20154-20160
- PubMed: 32757352
- DOI: https://doi.org/10.1002/anie.202008665
- Primary Citation of Related Structures:
6XHC, 6XHD, 6XHE, 6XHF - PubMed Abstract:
Phosphoramidates composed of an amino acid and a nucleotide analogue are critical metabolites of prodrugs, such as remdesivir. Hydrolysis of the phosphoramidate liberates the nucleotide, which can then be phosphorylated to become the pharmacologically active triphosphate. Enzymatic hydrolysis has been demonstrated, but a spontaneous chemical process may also occur. We measured the rate of enzyme-free hydrolysis for 17 phosphoramidates of ribonucleotides with amino acids or related compounds at pH 7.5. Phosphoramidates of proline hydrolyzed fast, with a half-life time as short as 2.4 h for Pro-AMP in ethylimidazole-containing buffer at 37 °C; 45-fold faster than Ala-AMP and 120-fold faster than Phe-AMP. Crystal structures of Gly-AMP, Pro-AMP, βPro-AMP and Phe-AMP bound to RNase A as crystallization chaperone showed how well the carboxylate is poised to attack the phosphoramidate, helping to explain this reactivity. Our results are significant for the design of new antiviral prodrugs.
Organizational Affiliation:
Institut für Organische Chemie, Universität Stuttgart, 70569, Stuttgart, Germany.