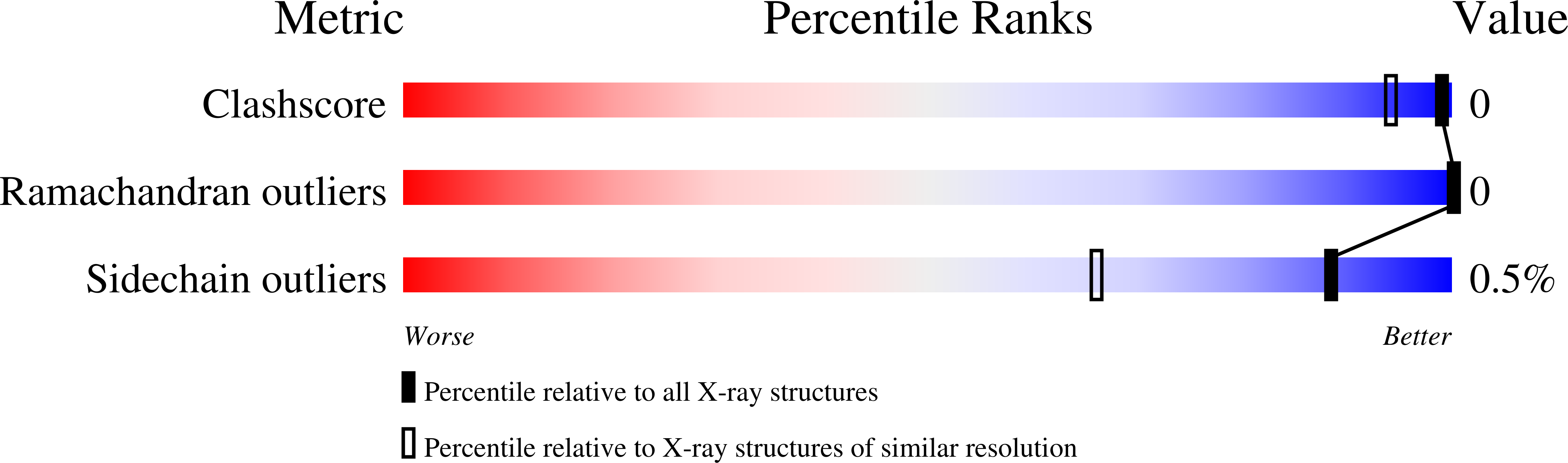Neutron structures of Leishmania mexicana triosephosphate isomerase in complex with reaction-intermediate mimics shed light on the proton-shuttling steps.
Kelpsas, V., Caldararu, O., Blakeley, M.P., Coquelle, N., Wierenga, R.K., Ryde, U., von Wachenfeldt, C., Oksanen, E.(2021) IUCrJ 8: 633-643
- PubMed: 34258011
- DOI: https://doi.org/10.1107/S2052252521004619
- Primary Citation of Related Structures:
7ABX, 7AZ3, 7AZ4, 7AZ9, 7AZA - PubMed Abstract:
Triosephosphate isomerase (TIM) is a key enzyme in glycolysis that catalyses the interconversion of glyceraldehyde 3-phosphate and dihydroxy-acetone phosphate. This simple reaction involves the shuttling of protons mediated by protolysable side chains. The catalytic power of TIM is thought to stem from its ability to facilitate the deprotonation of a carbon next to a carbonyl group to generate an enediolate intermediate. The enediolate intermediate is believed to be mimicked by the inhibitor 2-phosphoglycolate (PGA) and the subsequent enediol intermediate by phosphoglycolohydroxamate (PGH). Here, neutron structures of Leishmania mexicana TIM have been determined with both inhibitors, and joint neutron/X-ray refinement followed by quantum refinement has been performed. The structures show that in the PGA complex the postulated general base Glu167 is protonated, while in the PGH complex it remains deprotonated. The deuteron is clearly localized on Glu167 in the PGA-TIM structure, suggesting an asymmetric hydrogen bond instead of a low-barrier hydrogen bond. The full picture of the active-site protonation states allowed an investigation of the reaction mechanism using density-functional theory calculations.
Organizational Affiliation:
Department of Biology, Lund University, Sölvegatan 35, 223 62 Lund, Sweden.