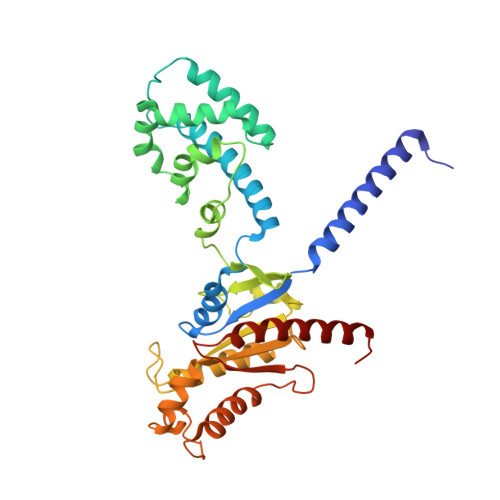
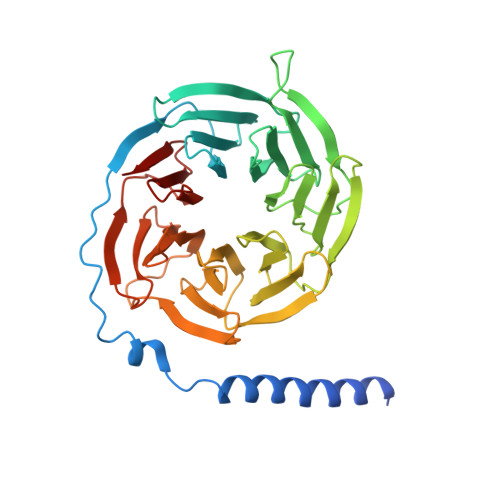
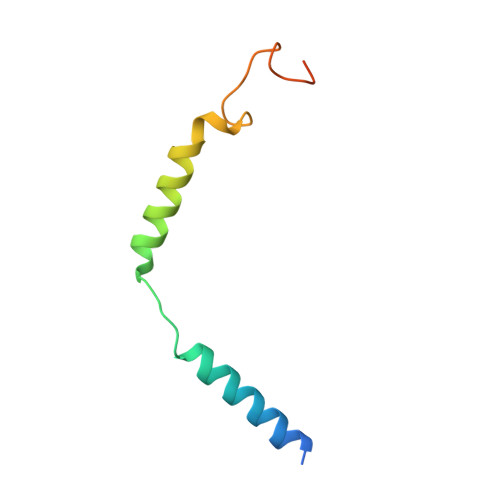
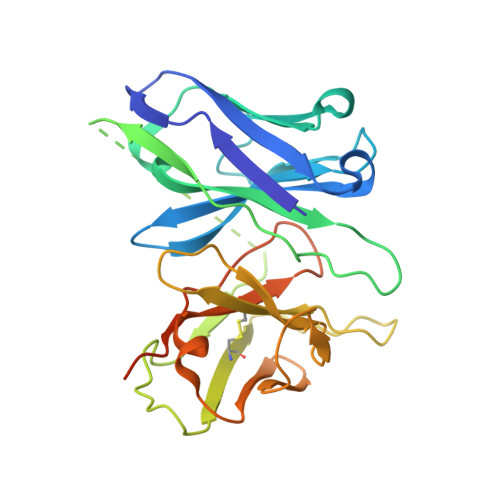
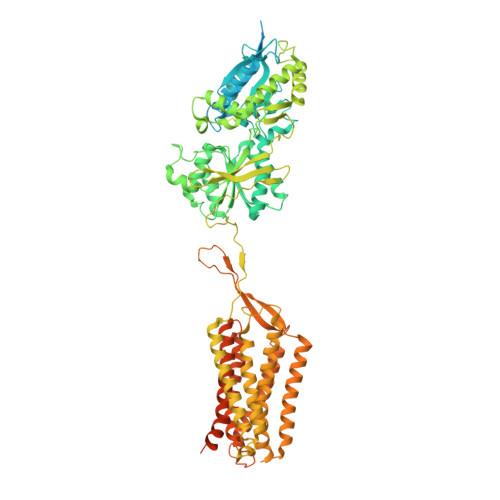
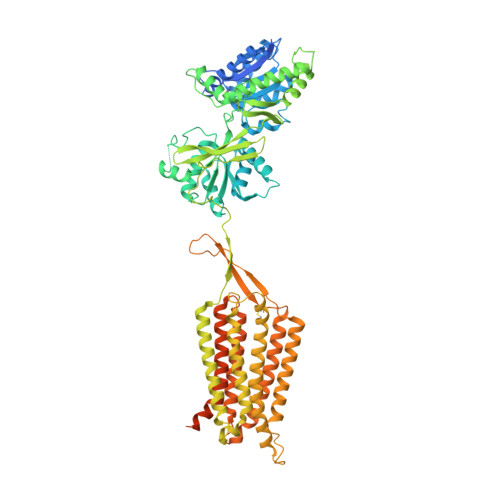
Structural basis of GABA B receptor-G i protein coupling.
Shen, C., Mao, C., Xu, C., Jin, N., Zhang, H., Shen, D.D., Shen, Q., Wang, X., Hou, T., Chen, Z., Rondard, P., Pin, J.P., Zhang, Y., Liu, J.(2021) Nature 594: 594-598
- PubMed: 33911284
- DOI: https://doi.org/10.1038/s41586-021-03507-1
- Primary Citation of Related Structures:
7EB2 - PubMed Abstract:
G-protein-coupled receptors (GPCRs) have central roles in intercellular communication 1,2 . Structural studies have revealed how GPCRs can activate G proteins. However, whether this mechanism is conserved among all classes of GPCR remains unknown. Here we report the structure of the class-C heterodimeric GABA B receptor, which is activated by the inhibitory transmitter GABA, in its active form complexed with G i1 protein. We found that a single G protein interacts with the GB2 subunit of the GABA B receptor at a site that mainly involves intracellular loop 2 on the side of the transmembrane domain. This is in contrast to the G protein binding in a central cavity, as has been observed with other classes of GPCR. This binding mode results from the active form of the transmembrane domain of this GABA B receptor being different from that of other GPCRs, as it shows no outside movement of transmembrane helix 6. Our work also provides details of the inter- and intra-subunit changes that link agonist binding to G-protein activation in this heterodimeric complex.
- ZJU-HUST Joint Laboratory of Cellular Signaling, Key Laboratory of Molecular Biophysics of MOE, International Research Center for Sensory Biology and Technology of MOST, College of Life Science and Technology, Huazhong University of Science and Technology (HUST), Wuhan, China.
Organizational Affiliation: