Dihydrodipicolinate synthase with pyruvate from the plant pathogen, Candidatus Liberibacter solanacearum
Gilkes, J.M., Frampton, R.A., Board, A., Sheen, C.R., Smith, G.R., Dobson, R.C.J.To be published.
Experimental Data Snapshot
Starting Model: experimental
View more details
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| 4-hydroxy-tetrahydrodipicolinate synthase | 295 | Candidatus Liberibacter solanacearum | Mutation(s): 0 Gene Names: dapA | 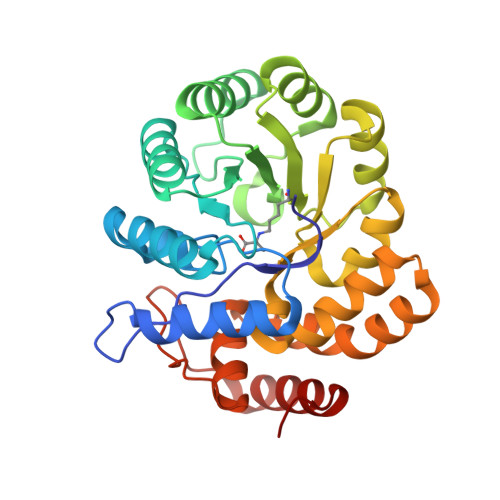 | |
UniProt | |||||
Find proteins for A0A0F4VK59 (Candidatus Liberibacter solanacearum) Go to UniProtKB: A0A0F4VK59 | |||||
Sequence AnnotationsExpand | |||||
| |||||
| Modified Residues 1 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Type | Formula | 2D Diagram | Parent |
| KPI Query on KPI | A, B, C, D, E A, B, C, D, E, F | L-PEPTIDE LINKING | C9 H16 N2 O4 |  | LYS |
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 101.777 | α = 90 |
| b = 133.581 | β = 100.55 |
| c = 155.988 | γ = 90 |
| Software Name | Purpose |
|---|---|
| REFMAC | refinement |
| pointless | data scaling |
| PHASER | phasing |
| XDS | data reduction |
| Funding Organization | Location | Grant Number |
|---|---|---|
| Not funded | -- |