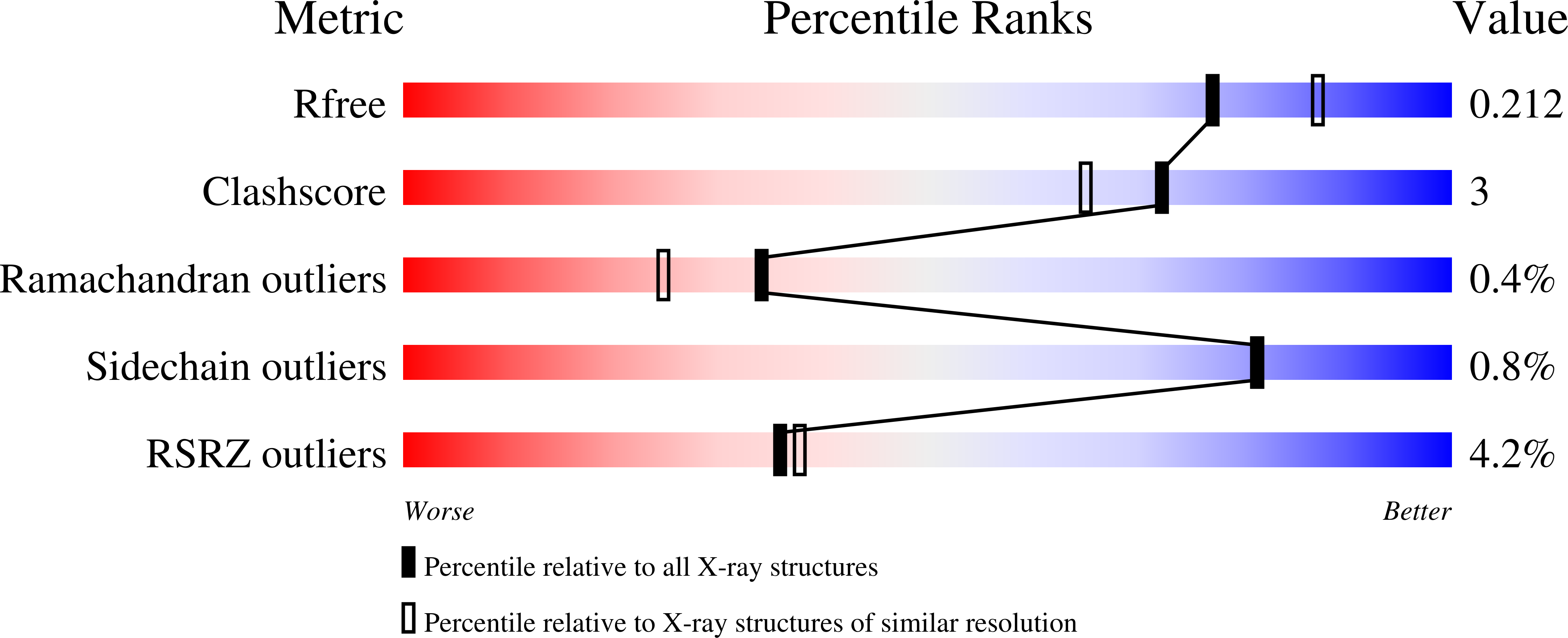
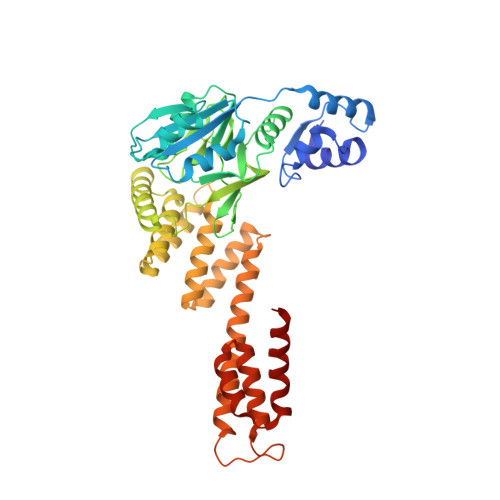
Structural and mechanistic investigations of protein S-glycosyltransferases.
Fujinami, D., Garcia de Gonzalo, C.V., Biswas, S., Hao, Y., Wang, H., Garg, N., Lukk, T., Nair, S.K., van der Donk, W.A.(2021) Cell Chem Biol 28: 1740-1749.e6
- PubMed: 34283964
- DOI: https://doi.org/10.1016/j.chembiol.2021.06.009
- Primary Citation of Related Structures:
7MSK, 7MSN, 7MSP - PubMed Abstract:
Attachment of sugars to nitrogen and oxygen in peptides is ubiquitous in biology, but glycosylation of sulfur atoms has only been recently described. Here, we characterize two S-glycosyltransferases SunS and ThuS that selectively glycosylate one of five Cys residues in their substrate peptides; substitution of this Cys with Ser results in a strong decrease in glycosylation activity. Crystal structures of SunS and ThuS in complex with UDP-glucose or a derivative reveal an unusual architecture in which a glycosyltransferase type A (GTA) fold is decorated with additional domains to support homodimerization. Dimer formation creates an extended cavity for the substrate peptide, drawing functional analogy with O-glycosyltransferases involved in cell wall biosynthesis. This extended cavity contains a sharp bend that may explain the site selectivity of the glycosylation because the target Cys is in a Gly-rich stretch that can accommodate the bend. These studies establish a molecular framework for understanding the unusual S-glycosyltransferases.
Organizational Affiliation:
Howard Hughes Medical Institute and Roger Adams Laboratory, Department of Chemistry, University of Illinois at Urbana-Champaign, 600 South Mathews Avenue, Urbana, IL 61801, USA.