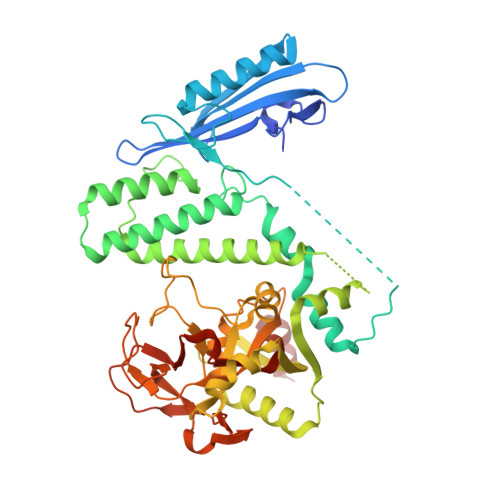
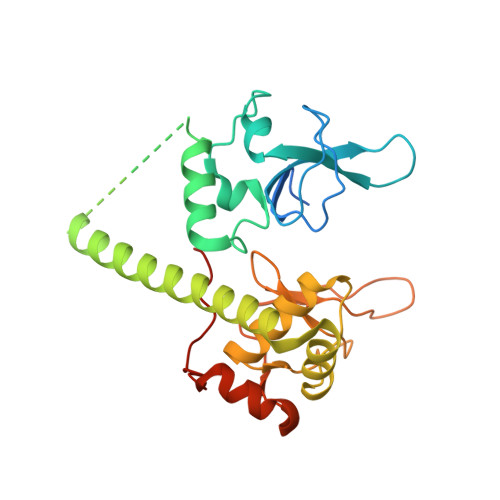
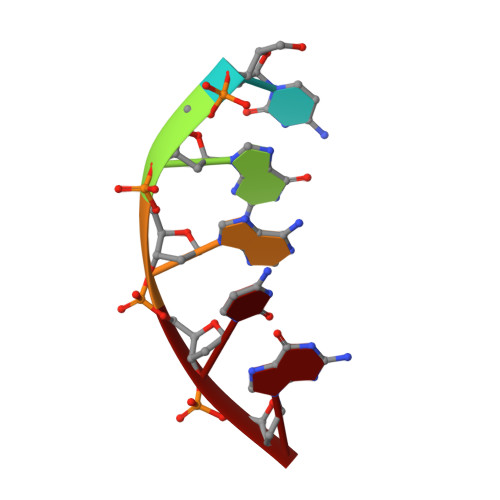
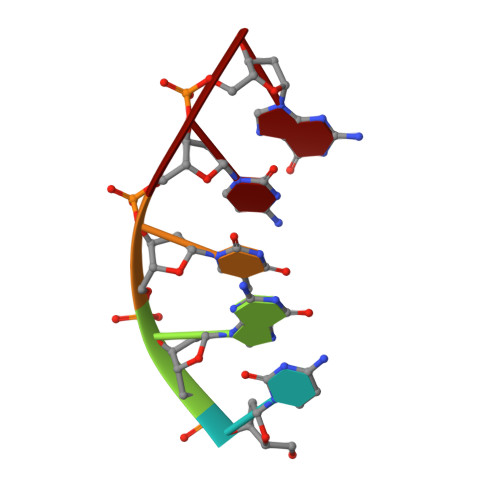
Captured snapshots of PARP1 in the active state reveal the mechanics of PARP1 allostery.
Rouleau-Turcotte, E., Krastev, D.B., Pettitt, S.J., Lord, C.J., Pascal, J.M.(2022) Mol Cell 82: 2939-2951.e5
- PubMed: 35793673
- DOI: https://doi.org/10.1016/j.molcel.2022.06.011
- Primary Citation of Related Structures:
7S68, 7S6H, 7S6M, 7S81 - PubMed Abstract:
PARP1 rapidly detects DNA strand break damage and allosterically signals break detection to the PARP1 catalytic domain to activate poly(ADP-ribose) production from NAD + . PARP1 activation is characterized by dynamic changes in the structure of a regulatory helical domain (HD); yet, there are limited insights into the specific contributions that the HD makes to PARP1 allostery. Here, we have determined crystal structures of PARP1 in isolated active states that display specific HD conformations. These captured snapshots and biochemical analysis illustrate HD contributions to PARP1 multi-domain and high-affinity interaction with DNA damage, provide novel insights into the mechanics of PARP1 allostery, and indicate how HD active conformations correspond to alterations in the catalytic region that reveal the active site to NAD + . Our work deepens the understanding of PARP1 catalytic activation, the dynamics of the binding site of PARP inhibitor compounds, and the mechanisms regulating PARP1 retention on DNA damage.
- Department of Biochemistry and Molecular Medicine, Université de Montréal, Montréal, QC H3T 1J4, Canada.
Organizational Affiliation: