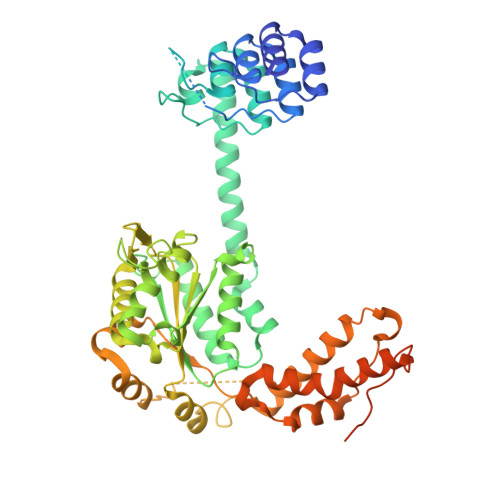
Unique structural features govern the activity of a human mitochondrial AAA+ disaggregase, Skd3.
Cupo, R.R., Rizo, A.N., Braun, G.A., Tse, E., Chuang, E., Gupta, K., Southworth, D.R., Shorter, J.(2022) Cell Rep 40: 111408-111408
- PubMed: 36170828
- DOI: https://doi.org/10.1016/j.celrep.2022.111408
- Primary Citation of Related Structures:
7TTR, 7TTS - PubMed Abstract:
The AAA+ protein, Skd3 (human CLPB), solubilizes proteins in the mitochondrial intermembrane space, which is critical for human health. Skd3 variants with defective protein-disaggregase activity cause severe congenital neutropenia (SCN) and 3-methylglutaconic aciduria type 7 (MGCA7). How Skd3 disaggregates proteins remains poorly understood. Here, we report a high-resolution structure of a Skd3-substrate complex. Skd3 adopts a spiral hexameric arrangement that engages substrate via pore-loop interactions in the nucleotide-binding domain (NBD). Substrate-bound Skd3 hexamers stack head-to-head via unique, adaptable ankyrin-repeat domain (ANK)-mediated interactions to form dodecamers. Deleting the ANK linker region reduces dodecamerization and disaggregase activity. We elucidate apomorphic features of the Skd3 NBD and C-terminal domain that regulate disaggregase activity. We also define how Skd3 subunits collaborate to disaggregate proteins. Importantly, SCN-linked subunits sharply inhibit disaggregase activity, whereas MGCA7-linked subunits do not. These advances illuminate Skd3 structure and mechanism, explain SCN and MGCA7 inheritance patterns, and suggest therapeutic strategies.
Organizational Affiliation:
Department of Biochemistry and Biophysics, University of Pennsylvania, Philadelphia, PA, USA; Pharmacology Graduate Group, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, USA.