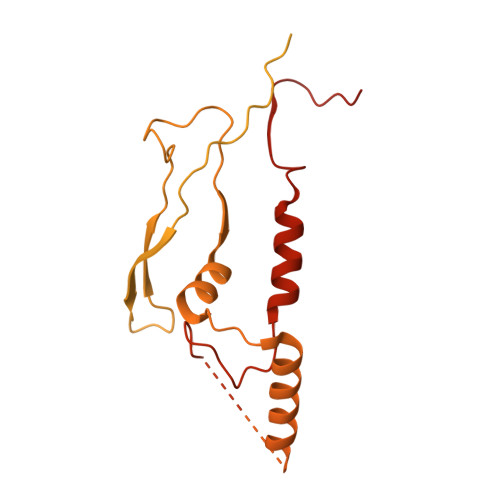
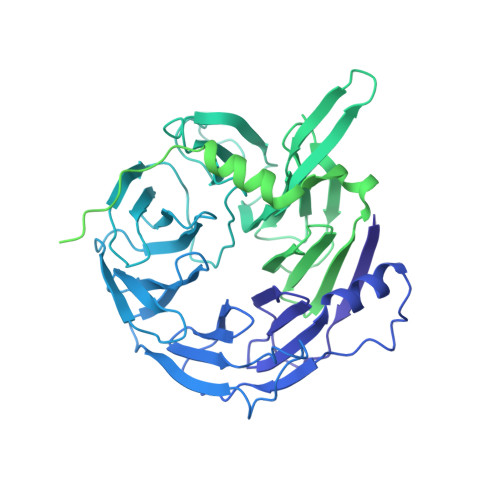
Structure of the Hir histone chaperone complex.
Kim, H.J., Szurgot, M.R., van Eeuwen, T., Ricketts, M.D., Basnet, P., Zhang, A.L., Vogt, A., Sharmin, S., Kaplan, C.D., Garcia, B.A., Marmorstein, R., Murakami, K.(2024) Mol Cell 84: 2601-2617.e12
- PubMed: 38925115
- DOI: https://doi.org/10.1016/j.molcel.2024.05.031
- Primary Citation of Related Structures:
8GHA, 8GHL, 8GHM, 8GHN, 8GIX - PubMed Abstract:
The evolutionarily conserved HIRA/Hir histone chaperone complex and ASF1a/Asf1 co-chaperone cooperate to deposit histone (H3/H4) 2 tetramers on DNA for replication-independent chromatin assembly. The molecular architecture of the HIRA/Hir complex and its mode of histone deposition have remained unknown. Here, we report the cryo-EM structure of the S. cerevisiae Hir complex with Asf1/H3/H4 at 2.9-6.8 Å resolution. We find that the Hir complex forms an arc-shaped dimer with a Hir1/Hir2/Hir3/Hpc2 stoichiometry of 2/4/2/4. The core of the complex containing two Hir1/Hir2/Hir2 trimers and N-terminal segments of Hir3 forms a central cavity containing two copies of Hpc2, with one engaged by Asf1/H3/H4, in a suitable position to accommodate a histone (H3/H4) 2 tetramer, while the C-terminal segments of Hir3 harbor nucleic acid binding activity to wrap DNA around the Hpc2-assisted histone tetramer. The structure suggests a model for how the Hir/Asf1 complex promotes the formation of histone tetramers for their subsequent deposition onto DNA.
Organizational Affiliation:
Department of Biochemistry and Biophysics, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA 19104, USA; Biochemistry and Molecular Biophysics Graduate Group, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA 19104, USA.