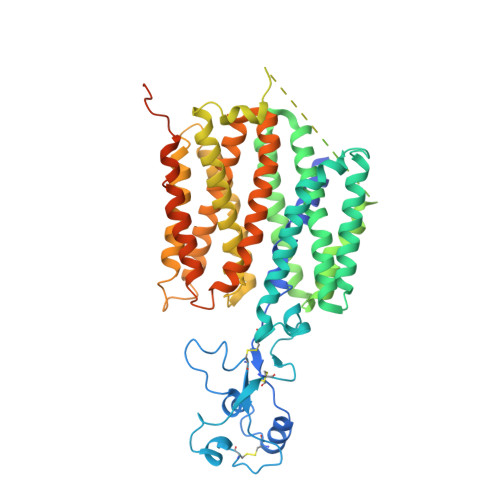
Structural insights into human organic cation transporter 1 transport and inhibition.
Zhang, S., Zhu, A., Kong, F., Chen, J., Lan, B., He, G., Gao, K., Cheng, L., Sun, X., Yan, C., Chen, L., Liu, X.(2024) Cell Discov 10: 30-30
- PubMed: 38485705
- DOI: https://doi.org/10.1038/s41421-024-00664-1
- Primary Citation of Related Structures:
8JTS, 8JTT, 8JTV, 8JTW, 8JTX, 8JTY, 8JTZ, 8JU0 - PubMed Abstract:
The human organic cation transporter 1 (hOCT1), also known as SLC22A1, is integral to hepatic uptake of structurally diversified endogenous and exogenous organic cations, influencing both metabolism and drug pharmacokinetics. hOCT1 has been implicated in the therapeutic dynamics of many drugs, making interactions with hOCT1 a key consideration in novel drug development and drug-drug interactions. Notably, metformin, the frontline medication for type 2 diabetes, is a prominent hOCT1 substrate. Conversely, hOCT1 can be inhibited by agents such as spironolactone, a steroid analog inhibitor of the aldosterone receptor, necessitating a deep understanding of hOCT1-drug interactions in the development of new pharmacological treatments. Despite extensive study, specifics of hOCT1 transport and inhibition mechanisms remain elusive at the molecular level. Here, we present cryo-electron microscopy structures of the hOCT1-metformin complex in three distinct conformational states - outward open, outward occluded, and inward occluded as well as substrate-free hOCT1 in both partially and fully open states. We also present hOCT1 in complex with spironolactone in both outward and inward facing conformations. These structures provide atomic-level insights into the dynamic metformin transfer process via hOCT1 and the mechanism by which spironolactone inhibits it. Additionally, we identify a 'YER' motif critical for the conformational flexibility of hOCT1 and likely other SLC22 family transporters. Our findings significantly advance the understanding of hOCT1 molecular function and offer a foundational framework for the design of new therapeutic agents targeting this transporter.
Organizational Affiliation:
State Key Laboratory of Membrane Biology, Tsinghua-Peking Center for Life Sciences, School of Pharmaceutical Sciences, Tsinghua University, Beijing, China.