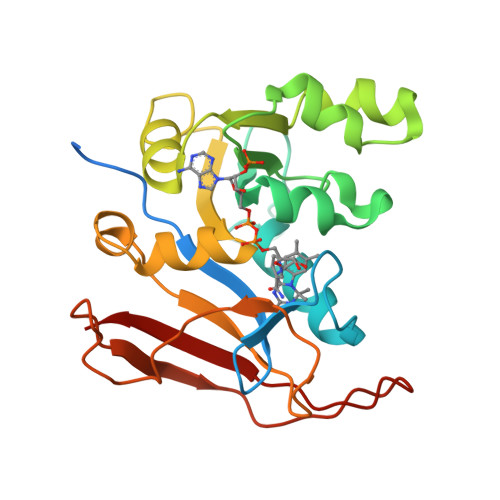
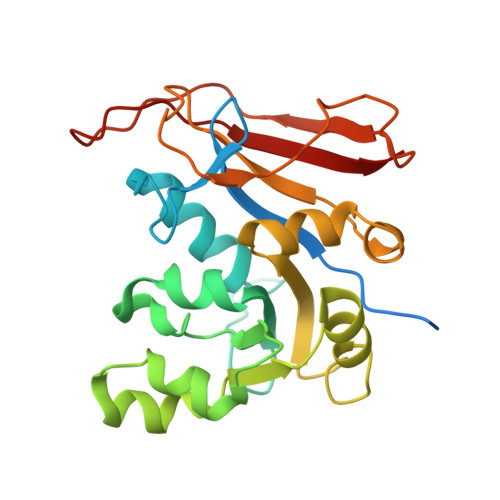
Identification of Innovative Folate Inhibitors Leveraging the Amino Dihydrotriazine Motif from Cycloguanil for Their Potential as Anti- Trypanosoma brucei Agents.
Francesconi, V., Rizzo, M., Pozzi, C., Tagliazucchi, L., Konchie Simo, C.U., Saporito, G., Landi, G., Mangani, S., Carbone, A., Schenone, S., Santarem, N., Tavares, J., Cordeiro-da-Silva, A., Costi, M.P., Tonelli, M.(2024) ACS Infect Dis 10: 2755-2774
- PubMed: 38953453
- DOI: https://doi.org/10.1021/acsinfecdis.4c00113
- Primary Citation of Related Structures:
8RHT, 8RHU, 8RHV, 8RHW, 8RHX, 8RHY - PubMed Abstract:
Folate enzymes, namely, dihydrofolate reductase (DHFR) and pteridine reductase (PTR1) are acknowledged targets for the development of antiparasitic agents against Trypanosomiasis and Leishmaniasis. Based on the amino dihydrotriazine motif of the drug Cycloguanil (Cyc), a known inhibitor of both folate enzymes, we have identified two novel series of inhibitors, the 2-amino triazino benzimidazoles ( 1 ) and 2-guanidino benzimidazoles ( 2 ), as their open ring analogues. Enzymatic screening was carried out against PTR1, DHFR, and thymidylate synthase (TS). The crystal structures of Tb DHFR and Tb PTR1 in complex with selected compounds experienced in both cases a substrate-like binding mode and allowed the rationalization of the main chemical features supporting the inhibitor ability to target folate enzymes. Biological evaluation of both series was performed against T. brucei and L. infantum and the toxicity against THP-1 human macrophages. Notably, the 5,6-dimethyl-2-guanidinobenzimidazole 2g resulted to be the most potent ( K i = 9 nM) and highly selective Tb DHFR inhibitor, 6000-fold over Tb PTR1 and 394-fold over h DHFR. The 5,6-dimethyl tricyclic analogue 1g , despite showing a lower potency and selectivity profile than 2g , shared a comparable antiparasitic activity against T. brucei in the low micromolar domain. The dichloro-substituted 2-guanidino benzimidazoles 2c and 2d revealed their potent and broad-spectrum antitrypanosomatid activity affecting the growth of T. brucei and L. infantum parasites. Therefore, both chemotypes could represent promising templates that could be valorized for further drug development.
Organizational Affiliation:
Department of Pharmacy, University of Genoa, viale Benedetto XV n.3, Genoa 16132, Italy.