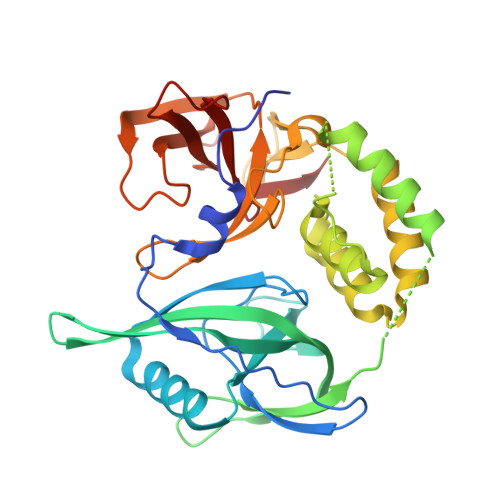
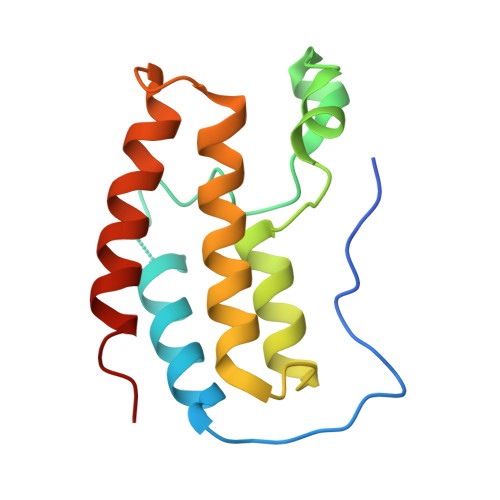
Design of a Cereblon construct for crystallographic and biophysical studies of protein degraders.
Kroupova, A., Spiteri, V.A., Rutter, Z.J., Furihata, H., Darren, D., Ramachandran, S., Chakraborti, S., Haubrich, K., Pethe, J., Gonzales, D., Wijaya, A.J., Rodriguez-Rios, M., Sturbaut, M., Lynch, D.M., Farnaby, W., Nakasone, M.A., Zollman, D., Ciulli, A.(2024) Nat Commun 15: 8885-8885
- PubMed: 39406745
- DOI: https://doi.org/10.1038/s41467-024-52871-9
- Primary Citation of Related Structures:
8RQ1, 8RQ8, 8RQ9, 8RQA, 8RQC, 9GAO - PubMed Abstract:
The ubiquitin E3 ligase cereblon (CRBN) is the target of therapeutic drugs thalidomide and lenalidomide and is recruited by most targeted protein degraders (PROTACs and molecular glues) in clinical development. Biophysical and structural investigation of CRBN has been limited by current constructs that either require co-expression with the adaptor DDB1 or inadequately represent full-length protein, with high-resolution structures of degrader ternary complexes remaining rare. We present the design of CRBN midi , a construct that readily expresses from E. coli with high yields as soluble, stable protein without DDB1. We benchmark CRBN midi for wild-type functionality through a suite of biophysical techniques and solve high-resolution co-crystal structures of its binary and ternary complexes with degraders. We qualify CRBN midi as an enabling tool to accelerate structure-based discovery of the next generation of CRBN based therapeutics.
Organizational Affiliation:
Centre for Targeted Protein Degradation, School of Life Sciences, University of Dundee, Dundee, DD1 5JJ, UK.