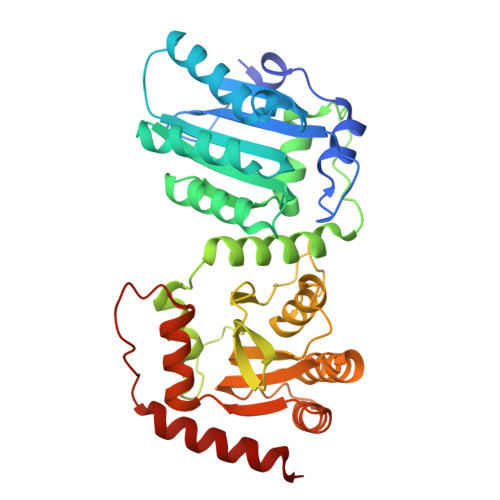
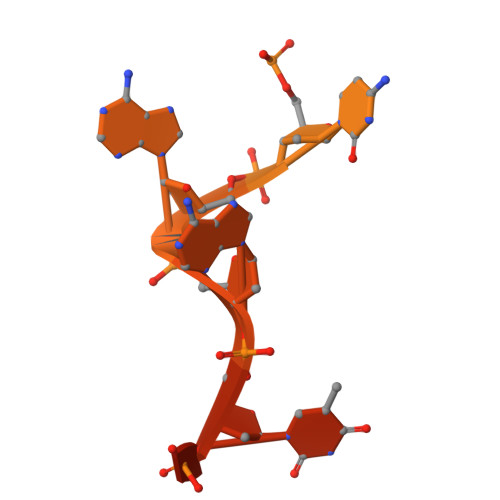
Molecular mechanism for regulating APOBEC3G DNA editing function by the non-catalytic domain.
Yang, H., Pacheco, J., Kim, K., Bokani, A., Ito, F., Ebrahimi, D., Chen, X.S.(2024) Nat Commun 15: 8773-8773
- PubMed: 39389938
- DOI: https://doi.org/10.1038/s41467-024-52671-1
- Primary Citation of Related Structures:
8TVC, 8TX4 - PubMed Abstract:
APOBEC3G, part of the AID/APOBEC cytidine deaminase family, is crucial for antiviral immunity. It has two zinc-coordinated cytidine-deaminase domains. The non-catalytic N-terminal domain strongly binds to nucleic acids, whereas the C-terminal domain catalyzes C-to-U editing in single-stranded DNA. The interplay between the two domains is not fully understood. Here, we show that DNA editing function of rhesus macaque APOBEC3G on linear and hairpin loop DNA is enhanced by AA or GA dinucleotide motifs present downstream in the 3'-direction of the target-C editing sites. The effective distance between AA/GA and the target-C sites is contingent on the local DNA secondary structure. We present two co-crystal structures of rhesus macaque APOBEC3G bound to ssDNA containing AA and GA, revealing the contribution of the non-catalytic domain in capturing AA/GA DNA. Our findings elucidate the molecular mechanism of APOBEC3G's cooperative function, which is critical for its antiviral role and its contribution to mutations in cancer genomes.
- Molecular and Computational Biology, Departments of Biological Sciences, University of Southern California, Los Angeles, CA, 90089, USA.
Organizational Affiliation: